Is Potassium Hydroxide Soluble In Water
News Leon
Mar 29, 2025 · 5 min read
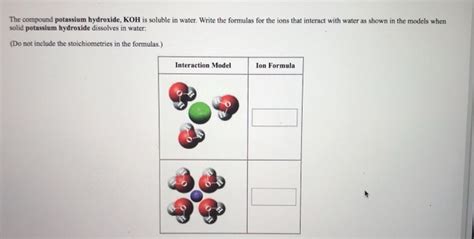
Table of Contents
Is Potassium Hydroxide Soluble in Water? A Deep Dive into Solubility and its Implications
Potassium hydroxide (KOH), also known as caustic potash, is a highly alkaline substance with a wide range of industrial and chemical applications. Understanding its solubility in water is crucial for its safe and effective use. This comprehensive article explores the solubility of potassium hydroxide in water, examining the factors influencing it, its implications in various applications, and safety considerations.
The Solubility of Potassium Hydroxide: A Definitive Answer
Yes, potassium hydroxide is highly soluble in water. This is a fundamental characteristic that dictates its behavior in various solutions and processes. The solubility is exothermic, meaning heat is released when KOH dissolves in water. This heat generation can be significant, especially when dissolving large quantities, and warrants careful handling.
Factors Affecting Solubility
While KOH's solubility in water is considerable, several factors can influence the exact degree of solubility:
-
Temperature: The solubility of potassium hydroxide in water increases significantly with increasing temperature. Hot water can dissolve substantially more KOH than cold water. This temperature dependence is a key consideration in many industrial processes using KOH solutions.
-
Concentration: While KOH is highly soluble, there's a limit to how much can be dissolved in a given volume of water at a specific temperature. At saturation point, no more KOH will dissolve, and any additional KOH added will remain as a solid. This saturation point is temperature-dependent.
-
Purity of KOH: The presence of impurities in the potassium hydroxide sample can affect its solubility. Impurities can hinder the dissolution process, leading to lower apparent solubility. High-purity KOH is essential for applications requiring precise control over concentration.
-
Presence of other solutes: The presence of other dissolved substances in the water can influence the solubility of KOH. Interactions between different ions in the solution can affect the equilibrium and the amount of KOH that can dissolve.
Understanding the Dissolution Process
The dissolution of potassium hydroxide in water is a complex process involving several steps:
-
Dissociation: When KOH is added to water, it dissociates into its constituent ions, potassium ions (K⁺) and hydroxide ions (OH⁻). This dissociation is virtually complete in aqueous solutions, making KOH a strong base.
-
Hydration: The potassium and hydroxide ions are then surrounded by water molecules. These water molecules form hydration shells around the ions, stabilizing them in the solution and reducing their tendency to recombine into solid KOH. The strong electrostatic interactions between the ions and water molecules drive the dissolution process.
-
Heat Generation: The process of hydration is exothermic, meaning it releases heat. This is due to the strong interactions between the ions and water molecules. The heat generated can be substantial, especially when dissolving large amounts of KOH, causing a significant increase in the temperature of the solution. This exothermic nature is a critical safety concern.
Applications Leveraging KOH's Solubility
The high solubility of potassium hydroxide in water is fundamental to its diverse applications across various industries. Here are some key examples:
1. Chemical Industry:
-
Production of Potassium Salts: KOH's solubility is essential in the production of various potassium salts, including potassium carbonate, potassium phosphate, and potassium sulfate. Reactions involving KOH typically occur in aqueous solutions, utilizing its high solubility to ensure efficient reaction kinetics.
-
Synthesis of Organic Compounds: KOH is a crucial reagent in many organic syntheses. Its solubility in water allows for the creation of aqueous solutions used in reactions such as saponification (soap making) and ester hydrolysis.
-
Electrolyte in Batteries: KOH's high solubility makes it a suitable electrolyte in alkaline batteries. Its aqueous solutions provide a medium for ion transport, facilitating the electrochemical reactions that power these batteries.
2. Food Industry:
-
pH Control: KOH is used as a pH regulator in various food processing applications. Its solubility allows for precise control of pH levels in food products. This is especially important in the production of certain types of processed foods where maintaining specific pH values is crucial for quality and safety.
-
Food Additives: In some limited instances, potassium hydroxide is employed as a food additive in small quantities. Its role here often centers around adjusting pH or acting as a processing aid. However, regulatory considerations and safety standards must be strictly adhered to.
3. Other Industries:
-
Water Treatment: KOH is used in some water treatment processes. Its solubility facilitates the adjustment of pH and the removal of certain impurities.
-
Cleaning and Disinfecting: Aqueous solutions of KOH are sometimes used for cleaning and disinfecting purposes, though other, less corrosive agents are often preferred.
-
Agriculture: Potassium hydroxide can be applied as a potassium source for plants; however, its solubility demands careful handling to avoid damage to plant tissues.
Safety Considerations: Handling KOH Solutions
Because of its high reactivity and the heat generated during dissolution, handling potassium hydroxide requires careful consideration of safety precautions:
-
Protective Equipment: Always wear appropriate personal protective equipment (PPE) when handling KOH, including gloves, eye protection, and a lab coat. Skin contact should be avoided at all costs.
-
Slow Addition: When dissolving KOH in water, always add the KOH to the water slowly, stirring gently. Never add water to KOH. Adding water to KOH can cause a violent exothermic reaction, leading to splashing and potential burns.
-
Ventilation: Dissolving KOH in water can generate heat and fumes. Ensure adequate ventilation to prevent inhalation of these fumes.
-
Waste Disposal: Dispose of KOH solutions according to local regulations. Never pour KOH down the drain without proper neutralization.
Conclusion: Solubility and its Significance
The high solubility of potassium hydroxide in water is a defining characteristic that underpins its extensive use in numerous applications. Understanding the factors affecting its solubility, the dissolution process itself, and the related safety concerns is critical for anyone working with this important chemical. From its role in chemical synthesis to its applications in food processing and other industries, KOH's solubility continues to play a vital role in many facets of modern life. However, careful handling and adherence to strict safety protocols are paramount to ensure safe and effective utilization. The exothermic nature of its dissolution and its corrosive properties necessitate proactive safety measures to prevent accidents and safeguard both personnel and the environment. This careful balance between utilizing the benefits of KOH's solubility and mitigating the risks associated with its handling is key to its responsible and effective application across diverse industries.
Latest Posts
Latest Posts
-
What Structure Is Found Only In Animal Cells
Mar 31, 2025
-
Which Is Not A Function Of The Cerebrospinal Fluid
Mar 31, 2025
-
A Vector Has Magnitude And Direction
Mar 31, 2025
-
Oxidation Number Of S In So2
Mar 31, 2025
-
Reduction Of An Aldehyde Produces A
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Potassium Hydroxide Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
