Is Melting Ice A Chemical Change
News Leon
Mar 15, 2025 · 5 min read
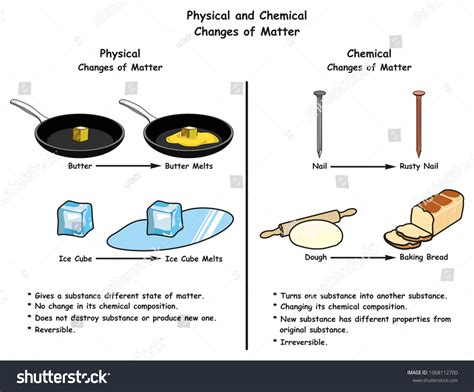
Table of Contents
Is Melting Ice a Chemical Change? Understanding Physical vs. Chemical Transformations
The question of whether melting ice constitutes a chemical change is a fundamental one in understanding the nature of matter and its transformations. The short answer is no, melting ice is a physical change. However, understanding why this is the case requires a deeper dive into the concepts of physical and chemical changes, the structure of water, and the implications of phase transitions. This article will explore these concepts in detail, providing a comprehensive answer suitable for students, educators, and anyone curious about the science behind ice melting.
Defining Physical and Chemical Changes
Before we delve into the specifics of ice melting, let's establish a clear definition of physical and chemical changes. This distinction is crucial for understanding the difference between the two.
Physical changes alter the form or appearance of a substance but do not change its chemical composition. These changes are often reversible. Examples include:
- Changes in state: Melting, freezing, boiling, condensation, sublimation (solid to gas), and deposition (gas to solid).
- Changes in shape: Cutting, bending, crushing.
- Changes in size: Dissolving (in some cases, as we'll discuss later).
In physical changes, the molecules themselves remain unchanged; only their arrangement or energy level changes.
Chemical changes, also known as chemical reactions, involve the rearrangement of atoms and molecules to form new substances with different properties. These changes are often irreversible or require significant energy input to reverse. Examples include:
- Burning: Combustion reactions involve oxygen and produce new substances like carbon dioxide and water.
- Rusting: Oxidation of iron in the presence of oxygen and water.
- Digestion: Complex molecules are broken down into simpler ones.
The Structure of Water and the Process of Melting
Water (H₂O) is a remarkable substance, and understanding its molecular structure is key to understanding its phase transitions. Each water molecule is composed of two hydrogen atoms covalently bonded to a single oxygen atom. This bonding creates a slightly polar molecule, meaning it has a slightly positive end (near the hydrogen atoms) and a slightly negative end (near the oxygen atom). This polarity leads to strong intermolecular forces, specifically hydrogen bonds, between water molecules.
These hydrogen bonds are responsible for many of water's unique properties, including its high boiling point, high surface tension, and its less dense solid state (ice). In ice, these hydrogen bonds create a relatively open, crystalline structure. When ice melts, the energy input (usually heat) overcomes these hydrogen bonds.
The Melting Process: A Microscopic View
As heat is applied to ice, the kinetic energy of the water molecules increases. This increased energy causes the molecules to vibrate more vigorously. Eventually, the vibrational energy becomes sufficient to break the hydrogen bonds holding the molecules in their fixed crystalline arrangement. The molecules then move more freely, transitioning from the rigid structure of ice to the more fluid structure of liquid water.
Crucially, the chemical composition of the water remains unchanged. The water molecules are still H₂O; only the arrangement and interactions between them have altered. This is the defining characteristic of a physical change.
Distinguishing between Dissolving and Melting
The process of dissolving can sometimes be confused with melting, especially when dealing with solids dissolving in liquids. However, they are fundamentally different processes.
Dissolving involves the separation of solute particles (the substance being dissolved) and their dispersion among solvent particles (the substance doing the dissolving). In some cases, this can involve the formation of new interactions between solute and solvent molecules, but the solute's chemical composition remains unchanged. For example, dissolving sugar in water results in a solution of sugar molecules dispersed in water; the sugar molecules themselves remain intact.
Melting, as discussed above, involves a change in state without altering the chemical composition. The key difference lies in the interaction between particles. Dissolving involves the interaction between different substances (solute and solvent), while melting involves the interaction between identical molecules of the same substance.
Addressing Potential Confusion: Chemical Changes Related to Ice
While the melting of ice itself is a physical change, it's important to note that chemical changes can occur in systems involving ice. For instance:
-
Reactions with salts: Adding salt to ice lowers its melting point. While the salt interacts with the water molecules, this is primarily a physical change (dissolution) and not a reaction that alters the chemical composition of either the salt or water. However, some salts could undergo chemical reactions under specific conditions.
-
Photochemical reactions on ice: Under certain conditions, UV radiation can induce photochemical reactions on the surface of ice, potentially leading to chemical changes in impurities present in the ice. However, the melting process itself is not responsible for these chemical reactions.
These examples highlight the complexity of real-world systems. While the fundamental process of ice melting is a physical change, secondary processes can occur that involve chemical transformations. It is vital to differentiate the primary physical change from any concurrent, independent chemical events.
Practical Implications and Real-World Examples
Understanding the distinction between physical and chemical changes has many practical implications. For example, in weather forecasting, understanding the phase transitions of water is crucial. The melting of snow and ice contributes to rising sea levels, impacting coastal communities globally. Similarly, in various industrial processes, such as ice-making or refrigeration, the knowledge that melting is a reversible physical change is vital for designing efficient systems.
Conclusion: Melting Ice Remains a Physical Change
In conclusion, the melting of ice is unequivocally a physical change. The process involves the breaking of intermolecular forces (hydrogen bonds) that maintain the crystalline structure of ice. While the arrangement and energy state of water molecules change, their chemical composition—H₂O—remains constant. While the melting of ice might be involved in a broader system where chemical reactions occur independently, the actual melting process itself is purely a physical transformation, clearly distinguished from the chemical changes that require the breaking and forming of chemical bonds. Understanding this distinction is essential for comprehending a vast range of scientific concepts and real-world phenomena.
Latest Posts
Latest Posts
-
Mountain Range That Separates Europe And Asia
Mar 15, 2025
-
16 Out Of 40 As A Percentage
Mar 15, 2025
-
Which Of The Following Is A True Solution
Mar 15, 2025
-
How Many Vertices Does A Rectangular Pyramid Have
Mar 15, 2025
-
Which Are Different Forms Of The Same Gene
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Melting Ice A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
