Is Hydrochloric Acid Or Water A Better Conductor
News Leon
Mar 20, 2025 · 6 min read
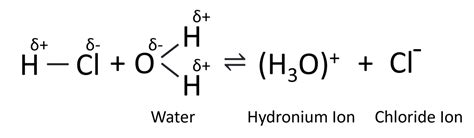
Table of Contents
Is Hydrochloric Acid or Water a Better Conductor of Electricity?
Understanding the conductive properties of different substances is crucial in various fields, from electrical engineering to chemistry. This article delves into the comparative conductivity of hydrochloric acid (HCl) and water (H₂O), explaining the underlying principles and factors that influence their ability to conduct electricity. We'll explore the role of ions, concentration, and temperature, providing a comprehensive analysis to answer the question: which is a better conductor, hydrochloric acid or water?
The Fundamentals of Electrical Conductivity
Before comparing HCl and H₂O, let's establish a basic understanding of electrical conductivity. A substance's ability to conduct electricity depends on its capacity to allow the movement of electrical charge carriers, typically ions or electrons. Materials are broadly categorized into conductors, insulators, and semiconductors based on their conductivity.
Conductors: These materials readily allow the flow of electric current due to the presence of freely moving charge carriers. Metals are classic examples, with their delocalized electrons facilitating easy current flow.
Insulators: These materials strongly resist the flow of electric current because they lack freely moving charge carriers. Most non-metals and many organic compounds fall into this category.
Semiconductors: These materials have conductivity that falls between conductors and insulators, often influenced by factors like temperature and doping. Silicon and germanium are prime examples, forming the basis of modern electronics.
Water: A Weak Conductor
Pure water (H₂O) is a poor conductor of electricity. While it contains both hydrogen and oxygen atoms, it doesn't readily allow the flow of electrical current. This is because water molecules themselves are electrically neutral. They don't readily release ions (charged particles) that can carry an electric charge. However, even pure water exhibits a very slight conductivity due to the self-ionization of water, where a small fraction of water molecules dissociate into hydronium ions (H₃O⁺) and hydroxide ions (OH⁻). This process is described by the equilibrium constant Kw, which is approximately 1 x 10⁻¹⁴ at 25°C. This minimal concentration of ions contributes to a negligible conductivity.
Factors Affecting Water's Conductivity
Several factors can influence the conductivity of water:
-
Dissolved Ions: The presence of dissolved salts, acids, or bases significantly increases the conductivity of water. These substances dissociate into ions, increasing the number of charge carriers and hence enhancing the current flow. Tap water, for example, typically has higher conductivity than distilled water due to dissolved minerals.
-
Temperature: Increasing temperature generally increases water's conductivity. Higher temperatures enhance the kinetic energy of molecules, leading to more frequent collisions and increased ionization. This effect, however, is relatively small compared to the impact of dissolved ions.
-
Purity: The purity of the water is a significant factor. Distilled water, being highly purified, exhibits lower conductivity than untreated water.
Hydrochloric Acid: A Strong Conductor
Hydrochloric acid (HCl) is a strong conductor of electricity. Unlike water, HCl is a strong acid that readily ionizes in solution. When HCl is dissolved in water, it dissociates completely into hydrogen ions (H⁺) and chloride ions (Cl⁻). These ions are highly mobile and readily carry an electric current. The high concentration of these ions is the primary reason for HCl's excellent conductivity.
Factors Affecting Hydrochloric Acid's Conductivity
Similar to water, several factors influence the conductivity of HCl:
-
Concentration: The concentration of HCl directly affects its conductivity. A higher concentration of HCl leads to a higher concentration of H⁺ and Cl⁻ ions, resulting in significantly improved conductivity. A dilute HCl solution will have lower conductivity than a concentrated one.
-
Temperature: As with water, temperature plays a role. Higher temperatures generally increase the mobility of ions, leading to slightly increased conductivity.
-
Presence of Other Ions: The presence of other dissolved ions, while not as impactful as the concentration of HCl itself, can still influence conductivity. These ions can interact with H⁺ and Cl⁻ ions, affecting their mobility.
Comparing Conductivity: HCl vs. Water
The significant difference in conductivity between HCl and water stems from the fundamental difference in their ionization properties. Water undergoes minimal self-ionization, producing only a few ions. In contrast, HCl completely dissociates into a high concentration of mobile ions when dissolved in water. This explains why HCl is a far better conductor of electricity than water.
The degree of conductivity can be quantified by measuring the solution's electrical conductivity using a conductivity meter. This instrument measures the ability of a solution to conduct an electric current and expresses the result in units like Siemens per meter (S/m) or microsiemens per centimeter (µS/cm). A significant difference in conductivity values between HCl and water would be readily apparent using such a measurement.
Practical Applications
The difference in conductivity between these two substances has numerous practical implications:
-
Electrolysis: HCl's high conductivity makes it suitable for electrolytic processes, where an electric current is used to drive chemical reactions. Water, having low conductivity, is less suitable for these applications without the addition of electrolytes.
-
Batteries: Electrolytes in batteries rely on the movement of ions to conduct electricity. HCl, due to its strong ionization, could potentially be used in certain battery designs although safety concerns would need to be carefully addressed.
-
Corrosion: The presence of ions in solutions, as found in HCl, facilitates the corrosion of metals. The high concentration of ions in HCl enhances the electrochemical reactions that cause corrosion.
-
Industrial Processes: Many industrial processes, such as electroplating and metal refining, leverage the conductive properties of solutions. The selection of a solution with appropriate conductivity, like HCl in some cases, is critical to the efficiency and safety of these processes.
Safety Considerations
It's crucial to emphasize the safety precautions associated with handling both water and especially hydrochloric acid. Hydrochloric acid is a highly corrosive substance that can cause severe burns and injuries. Appropriate safety measures, including protective eyewear, gloves, and lab coats, should always be employed when working with HCl. Proper ventilation is also essential to avoid inhalation of the acid fumes.
Conclusion: Hydrochloric Acid is the Superior Conductor
In summary, hydrochloric acid (HCl) is a far better conductor of electricity than water (H₂O). This difference arises from the complete ionization of HCl in solution, producing a high concentration of mobile ions that readily carry an electric current. Water, on the other hand, exhibits minimal self-ionization, resulting in significantly lower conductivity. The concentration of the acid, temperature, and the presence of other ions all play a role in influencing the conductivity of both substances. Understanding these principles is vital in various applications and safety considerations related to the handling of these chemicals.
Latest Posts
Latest Posts
-
Which Is An Interconnection Of Food Chains In An Ecosystem
Mar 21, 2025
-
One Horse Power Is Equal To How Many Watts
Mar 21, 2025
-
Biped Is To Quadruped As Ostrich Is To
Mar 21, 2025
-
How Many Seconds Are In 1 Hr
Mar 21, 2025
-
The Small Space Between Neurons Is Called
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Is Hydrochloric Acid Or Water A Better Conductor . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
