Is Evaporation Of Water A Physical Change
News Leon
Mar 16, 2025 · 5 min read
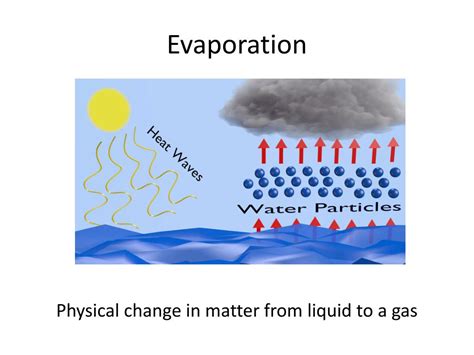
Table of Contents
Is the Evaporation of Water a Physical Change? A Deep Dive
The simple answer is yes, the evaporation of water is a physical change. But understanding why requires a deeper look into the nature of physical changes, the properties of water, and the process of evaporation itself. This article will explore these aspects, clarifying the distinction between physical and chemical changes and demonstrating unequivocally why evaporation fits the definition of a physical change.
Understanding Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. The molecules of the substance remain the same; they simply rearrange or move differently. Think of it like this: you can change the shape of clay, but it's still clay. Examples of physical changes include:
- Melting: Ice (solid water) turning into liquid water.
- Freezing: Liquid water turning into ice.
- Boiling: Liquid water turning into water vapor (steam).
- Condensation: Water vapor turning into liquid water.
- Dissolving: Salt dissolving in water.
In each of these cases, the fundamental chemical makeup of the substance doesn't change. Water remains H₂O throughout the transformation.
The Chemical Structure of Water (H₂O)
To fully appreciate why evaporation is a physical change, we need to understand the structure of water. Water is a molecule composed of two hydrogen atoms covalently bonded to a single oxygen atom. This covalent bond is a strong chemical bond, meaning it requires a significant amount of energy to break. Crucially, during evaporation, these covalent bonds within the water molecule itself remain intact.
This is the key differentiating factor between a physical and a chemical change. A chemical change involves the breaking and forming of chemical bonds, resulting in new substances with different properties. For example, burning wood is a chemical change because the cellulose in the wood reacts with oxygen to form carbon dioxide, water, and ash – completely different substances.
The Process of Evaporation: A Molecular Perspective
Evaporation is a surface phenomenon. It occurs when water molecules at the surface gain enough kinetic energy (energy of motion) to overcome the intermolecular forces holding them together in the liquid state. These intermolecular forces, such as hydrogen bonds, are weaker than covalent bonds.
Here's a breakdown of what happens at a molecular level:
- Kinetic Energy: Water molecules are constantly moving, colliding with each other and the container walls. The temperature of the water reflects the average kinetic energy of these molecules.
- Surface Molecules: Molecules at the surface of the liquid experience fewer intermolecular attractions than those in the bulk of the liquid.
- Escape: When a surface molecule gains sufficient kinetic energy from collisions or heat absorption, it can overcome the weaker intermolecular forces and escape into the gaseous phase. This is evaporation.
- No Bond Breaking: Importantly, the covalent bonds within the water molecule (O-H bonds) remain unbroken. The water molecule escapes as a whole entity, albeit now in the gaseous state.
Distinguishing Evaporation from Other Water-Related Changes
It's essential to distinguish evaporation from other processes that might seem similar but involve chemical changes:
- Electrolysis of Water: This is a chemical change where water is decomposed into hydrogen and oxygen gas using an electric current. Here, the covalent bonds within the water molecule are broken, creating entirely new substances.
- Reactions with Water: Water can participate in chemical reactions, such as the reaction between water and sodium metal, which produces hydrogen gas and sodium hydroxide. Again, this involves the breaking and formation of chemical bonds, resulting in new chemical species.
Evaporation, however, remains a purely physical process. The water molecule remains intact throughout the transformation from liquid to gas.
Evidence for Evaporation as a Physical Change
Several observations support the classification of evaporation as a physical change:
- Reversibility: Evaporation is a reversible process. The water vapor produced can be condensed back into liquid water through cooling, demonstrating that the chemical composition remains unchanged.
- No New Substance Formed: No new substance is formed during evaporation. The water remains H₂O, only its physical state changes.
- Energy Changes: While energy is absorbed during evaporation (endothermic process), this energy is used to overcome intermolecular forces, not to break chemical bonds. This is typical of physical changes.
Practical Applications and Real-World Examples
Understanding evaporation as a physical change has numerous practical applications:
- Weather Patterns: Evaporation plays a critical role in the water cycle, driving weather patterns and climate.
- Cooling Systems: Evaporation is utilized in cooling systems like sweating and evaporative coolers, exploiting the endothermic nature of the process.
- Desalination: Evaporation can be used to separate salt from seawater, creating fresh water. While this involves energy-intensive processes, the underlying change in the water itself remains physical.
- Drying: Evaporation is essential in various drying processes, such as drying clothes or food.
Conclusion: Evaporation is a Physical Change, Plain and Simple
In summary, the evaporation of water is unequivocally a physical change. The water molecule's chemical composition (H₂O) remains unchanged throughout the process. Only the physical state of the water changes, from liquid to gas, due to the absorption of energy and the overcoming of intermolecular forces. Understanding this distinction is crucial for grasping the fundamentals of chemistry and numerous natural phenomena and technological applications. The reversibility of the process, the lack of new substance formation, and the energy changes involved all strongly support this classification. Therefore, any assertion to the contrary is fundamentally incorrect.
Latest Posts
Latest Posts
-
What Percent Of 68 Is 17
Mar 17, 2025
-
Which Bond Is The Most Polar
Mar 17, 2025
-
Burning Of Candle Is Chemical Change
Mar 17, 2025
-
An Earth Satellite Moves In A Circular Orbit
Mar 17, 2025
-
What Is The Value Of K In Physics
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Evaporation Of Water A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
