Is Carbon Or Nitrogen More Electronegative
News Leon
Mar 24, 2025 · 5 min read
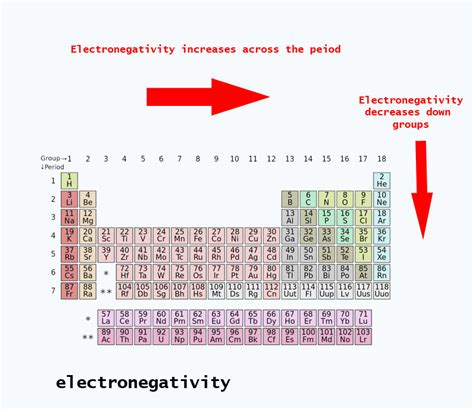
Table of Contents
Is Carbon or Nitrogen More Electronegative? A Deep Dive into Electronegativity
Electronegativity, a fundamental concept in chemistry, dictates the power of an atom to attract electrons within a chemical bond. Understanding electronegativity is crucial for predicting molecular geometry, polarity, and reactivity. This article will delve into a detailed comparison of carbon and nitrogen's electronegativity, exploring the underlying reasons for the difference and its implications in various chemical contexts. We'll examine the periodic trends influencing electronegativity, the consequences of electronegativity differences in bonding, and provide practical examples to solidify your understanding.
Understanding Electronegativity
Electronegativity isn't a directly measurable property like mass or charge. Instead, it's a relative measure, typically expressed using the Pauling scale, where fluorine, the most electronegative element, is assigned a value of 4.0. Other elements are then assigned values relative to fluorine. The higher the electronegativity value, the stronger an atom's pull on shared electrons in a covalent bond. This pull creates a dipole moment, where one end of the bond carries a slightly negative charge (δ-), and the other end carries a slightly positive charge (δ+).
Several factors influence an atom's electronegativity:
- Nuclear Charge: A higher nuclear charge (more protons) exerts a stronger attractive force on electrons.
- Atomic Radius: Smaller atoms have electrons closer to the nucleus, experiencing a stronger pull.
- Shielding Effect: Inner electrons shield outer electrons from the full nuclear charge, reducing the effective nuclear charge felt by the valence electrons.
Comparing Carbon and Nitrogen
Both carbon and nitrogen are nonmetals located in the second period of the periodic table. However, they occupy different groups, influencing their electronegativity:
- Carbon (C): Group 14, electronegativity ≈ 2.55
- Nitrogen (N): Group 15, electronegativity ≈ 3.04
Nitrogen is unequivocally more electronegative than carbon. This difference, though seemingly small, has significant consequences for their chemical behavior and the properties of the compounds they form.
The Reason for the Difference: A Closer Look
The difference in electronegativity between carbon and nitrogen stems primarily from their effective nuclear charge and atomic radius.
Effective Nuclear Charge: While nitrogen has one more proton than carbon, increasing the nuclear charge, the added electron goes into the same 2p subshell. The shielding effect is roughly similar for both atoms, meaning nitrogen experiences a greater effective nuclear charge than carbon. This stronger effective nuclear charge allows nitrogen to attract electrons more strongly.
Atomic Radius: Nitrogen has a slightly smaller atomic radius than carbon. The valence electrons in nitrogen are closer to the nucleus, experiencing a stronger attractive force. This smaller size contributes to nitrogen's higher electronegativity.
Implications of the Electronegativity Difference
The greater electronegativity of nitrogen significantly impacts the properties of molecules containing both carbon and nitrogen atoms.
1. Bond Polarity
In C-N bonds, the shared electrons are pulled more strongly towards the nitrogen atom, creating a polar bond. Nitrogen carries a partial negative charge (δ-), and carbon carries a partial positive charge (δ+). The magnitude of this polarity depends on the surrounding atoms and the overall molecular structure.
2. Molecular Geometry and Dipole Moment
The polarity of individual bonds influences the overall molecular polarity. In molecules like methylamine (CH3NH2), the presence of polar C-N bonds, along with the polar N-H bonds, results in a net dipole moment, making the molecule polar. This polarity affects its solubility in polar solvents (like water) and its interactions with other polar molecules.
3. Reactivity
The higher electronegativity of nitrogen often makes it a better nucleophile (electron-pair donor) compared to carbon in similar chemical environments. This increased nucleophilicity plays a critical role in many organic reactions, particularly those involving nitrogen-containing functional groups like amines and amides.
4. Acid-Base Properties
The electronegativity difference significantly affects the acid-base behavior of molecules containing carbon and nitrogen. For instance, amines (containing an N-H bond) are weakly basic due to nitrogen's ability to accept a proton (H+). The lone pair of electrons on nitrogen is attracted to the proton, forming a new N-H bond. Carbon, being less electronegative, doesn't exhibit such strong basic properties.
5. Hydrogen Bonding
Nitrogen's higher electronegativity enables it to participate in hydrogen bonding, a strong intermolecular force. This is particularly important in biological molecules like proteins and nucleic acids, where hydrogen bonding between nitrogen atoms (e.g., in peptide bonds and base pairs) contributes significantly to their structure and function.
Examples in Organic Chemistry
The electronegativity difference between carbon and nitrogen is fundamentally important in a myriad of organic compounds and reactions.
1. Amides: The carbonyl group (C=O) in amides exhibits a significant dipole moment due to the electronegativity difference between carbon and oxygen. The nitrogen atom, also more electronegative than carbon, further contributes to the overall polar character of the amide functional group. This polarity influences the physical and chemical properties of amides.
2. Amines: Amines are basic compounds. The nitrogen atom's lone pair readily accepts a proton, making the molecule basic. The electronegativity difference between nitrogen and the attached carbon atoms contributes to the strength of this basicity.
3. Nitriles: The triple bond between carbon and nitrogen in nitriles (R-C≡N) is highly polar due to nitrogen's greater electronegativity. This polarity makes nitriles useful in various synthetic applications.
4. Amino Acids: Amino acids, the building blocks of proteins, contain both amino (-NH2) and carboxyl (-COOH) groups. The nitrogen atom in the amino group is more electronegative than the carbon atom, making it a significant factor in the overall polarity and properties of amino acids, thereby influencing protein structure and function.
Conclusion: Nitrogen's Superior Electronegativity
In conclusion, nitrogen is demonstrably more electronegative than carbon. This difference stems from nitrogen's higher effective nuclear charge and smaller atomic radius, enabling it to attract shared electrons more strongly. This seemingly small difference has profound implications across various chemical contexts, impacting bond polarity, molecular geometry, reactivity, acid-base properties, hydrogen bonding, and the properties of countless organic molecules. Understanding this fundamental electronegativity difference is crucial for comprehending the behavior and properties of a vast array of organic and inorganic compounds. From simple molecules to complex biomolecules, the electronegativity contrast between carbon and nitrogen plays a pivotal role in shaping the chemical world around us.
Latest Posts
Latest Posts
-
Which Of The Following Gives Rise To Epithelial Tissues Only
Mar 29, 2025
-
Arrange The Following Carboxylic Acids In Order Of Acidity
Mar 29, 2025
-
What Is The Mass Of 1 Molecule Of Water
Mar 29, 2025
-
What Does Isalpha Do In Python
Mar 29, 2025
-
What Is The Horizontal Axis Called
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is Carbon Or Nitrogen More Electronegative . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
