Is Boron Solid Liquid Or Gas
News Leon
Mar 19, 2025 · 5 min read
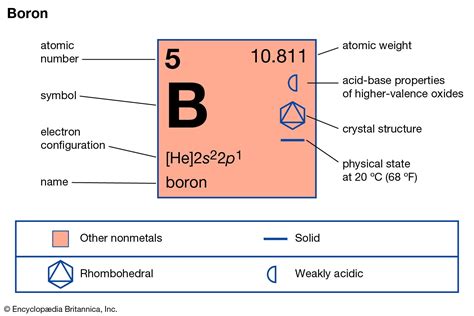
Table of Contents
Is Boron Solid, Liquid, or Gas? A Deep Dive into Boron's Properties
Boron, a metalloid element with the symbol B and atomic number 5, presents a fascinating case study in the states of matter. While many elements clearly exist as solids, liquids, or gases under standard conditions, boron's behavior is more nuanced. This article will explore boron's physical properties, its phase transitions, and the factors influencing its state, ultimately answering the question: is boron solid, liquid, or gas? We'll also touch upon boron's unique characteristics and applications, linking the understanding of its state to its practical uses.
Understanding the States of Matter
Before delving into boron's specific properties, let's briefly review the three fundamental states of matter: solid, liquid, and gas. These states are defined by the strength of intermolecular forces and the arrangement of atoms or molecules.
-
Solid: In a solid state, atoms or molecules are tightly packed in a highly ordered arrangement, characterized by strong intermolecular forces. This results in a fixed shape and volume. Solids resist deformation and maintain their structure.
-
Liquid: Liquids have weaker intermolecular forces than solids, allowing atoms or molecules to move more freely. They possess a definite volume but take the shape of their container. Liquids are relatively incompressible.
-
Gas: Gases have the weakest intermolecular forces, allowing atoms or molecules to move independently and randomly. Gases occupy the entire volume of their container and are highly compressible.
Boron: A Metalloid with Unique Properties
Boron occupies a unique position in the periodic table as a metalloid. Metalloids possess properties intermediate between those of metals and nonmetals. This means their behavior can be somewhat unpredictable, and their physical properties can vary depending on conditions. Boron is a hard, brittle, and crystalline solid under standard conditions (room temperature and atmospheric pressure). However, its behavior at higher temperatures warrants further investigation.
Boron's Crystalline Structure
Boron's crystalline structure is a significant factor determining its solid state at standard conditions. Unlike many other elements with simple crystal structures, boron forms complex structures. The most common allotrope (form) of boron is an icosahedral structure, where 12 boron atoms are arranged in a nearly spherical cluster. These icosahedra are then connected to each other through additional boron atoms, leading to a complex three-dimensional network. This robust and intricate structure contributes to boron's high hardness and high melting point.
Boron's High Melting Point
Boron boasts an exceptionally high melting point of approximately 2076 °C (3769 °F). This high melting point directly relates to the strong covalent bonds between boron atoms within its complex crystalline structure. To transition from a solid to a liquid, these strong bonds need to be overcome, requiring a substantial input of energy in the form of heat. This makes it difficult to melt boron under normal conditions.
Boron's Boiling Point
Similar to its high melting point, boron exhibits a very high boiling point, estimated to be around 3927 °C (7101 °F). Again, this is a direct consequence of the strong covalent bonds between boron atoms. A significant amount of energy is necessary to break these bonds and allow the boron atoms to transition into the gaseous phase.
Is Boron Ever Liquid?
While boron is predominantly known as a solid, it does exist in a liquid state at extremely high temperatures. However, achieving and maintaining these temperatures is challenging and requires specialized equipment. In essence, while boron's liquid form exists, it’s not readily observable under typical circumstances.
The Challenges of Liquifying Boron
The high melting point of boron presents significant practical challenges in producing liquid boron. Achieving and maintaining temperatures above 2076 °C requires high-power furnaces and precise temperature control. Even then, the high reactivity of liquid boron at these temperatures necessitates the use of inert atmospheres to prevent reactions with the surrounding environment.
Techniques for Liquifying Boron
Researchers utilize specialized techniques such as arc melting, zone refining, and float-zone crystallization to melt and purify boron. These techniques involve using intense heat sources and controlled environments to overcome the energetic barriers associated with boron's phase transition. These methods are complex and expensive, limiting widespread application.
Boron's Gaseous State
Boron's gaseous state is even less commonly observed than its liquid state. The transition to the gaseous phase requires even more energy than the liquid phase transition. Under typical conditions, boron atoms have very low probability of existing in a gaseous state. At the extremely high temperatures required to boil boron, the atoms would exist as individual atoms, rather than as molecules.
Applications of Boron and its States
Despite the challenges associated with its melting and vaporization, boron finds applications in various fields, leveraging its unique properties:
-
Solid Boron: Solid boron is utilized in materials science for creating high-strength alloys, semiconductors, and high-temperature ceramics. Its hardness and chemical resistance make it suitable for such demanding applications.
-
Boron Compounds: Many boron compounds, rather than elemental boron itself, find practical applications. Borax, boric acid, and boron carbide are among the extensively used boron compounds. These compounds find their use in detergents, insecticides, nuclear reactors, and many more areas.
Conclusion: Boron Remains Primarily Solid
In summary, while boron can technically exist in liquid and gaseous states at extremely high temperatures, it predominantly exists as a solid under standard conditions and in most practical applications. Its intricate crystalline structure, high melting and boiling points, and strong covalent bonds all contribute to its characteristic solid state. The challenges in achieving and handling liquid or gaseous boron limit its applications in these states, with the majority of its uses stemming from its solid forms and its diverse compounds. The complexities surrounding boron's phase transitions highlight its unique characteristics as a metalloid and underscore the significance of understanding the interplay between atomic structure, intermolecular forces, and the resulting state of matter.
Latest Posts
Latest Posts
-
Computer Programs Are Also Known As
Mar 20, 2025
-
Atp Is Called The Energy Currency Of The Cell Because
Mar 20, 2025
-
Which Of The Following Is An Even Function
Mar 20, 2025
-
Four Capacitors Are Connected As Shown In The Figure
Mar 20, 2025
-
What Energy Tranformation Happens In A Motot
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Boron Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
