Is A Metaloids Considered A Noble Gas
News Leon
Mar 26, 2025 · 5 min read
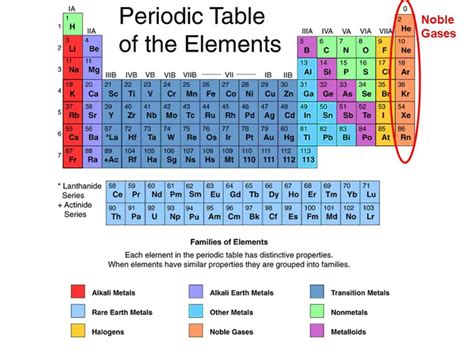
Table of Contents
Are Metalloids Considered Noble Gases? A Deep Dive into Elemental Classification
The periodic table, a cornerstone of chemistry, organizes elements based on their properties. Understanding the distinctions between different element categories, like metals, nonmetals, and metalloids, is crucial for comprehending chemical behavior. A frequent question arises regarding the classification of metalloids: are metalloids considered noble gases? The short answer is a resounding no. However, a more detailed exploration of the properties of both metalloids and noble gases reveals why this is the case and highlights the fundamental differences between these two distinct groups.
Understanding Noble Gases: The Inert Giants
Noble gases, also known as inert gases, occupy Group 18 of the periodic table. Their defining characteristic is their exceptional stability. This stability stems from their electron configuration: they possess a full outermost electron shell (valence shell), typically containing eight electrons (except for helium, which has two). This complete valence shell renders them extremely unreactive, hence their designation as "inert."
Key Properties of Noble Gases:
- Extreme Inertness/Low Reactivity: This is their most prominent feature. They rarely form chemical compounds, owing to their stable electron configuration.
- Colorless, Odorless, and Tasteless Gases under Standard Conditions: They exist as monatomic gases at room temperature and standard atmospheric pressure.
- Low Boiling Points: Reflecting weak interatomic forces due to their non-polar nature.
- Poor Conductors of Electricity and Heat: A consequence of their full valence electron shells.
Examples of noble gases include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Although exceptionally unreactive, some heavier noble gases, like xenon, have been known to form compounds under very specific conditions, challenging the long-held notion of their complete inertness. However, even these exceptions are rare and require extreme conditions.
Exploring Metalloids: The Semiconductors
Metalloids, also known as semimetals, occupy a diagonal band on the periodic table separating metals and nonmetals. They possess properties intermediate between metals and nonmetals, exhibiting characteristics of both. This makes them particularly useful in various technological applications.
Key Properties of Metalloids:
- Variable Conductivity: This is a defining characteristic. Metalloids are semiconductors, meaning their electrical conductivity varies with temperature and other factors. They conduct electricity better than nonmetals but less effectively than metals.
- Brittle Solids: Unlike most metals, metalloids are typically brittle and fracture easily.
- Metallic Luster (sometimes): Some metalloids exhibit a metallic sheen, while others appear duller.
- Intermediate Reactivity: Their reactivity is significantly higher than that of noble gases, falling between metals and nonmetals. They can form compounds with various elements under specific conditions.
- Semiconducting Properties: This is perhaps their most important attribute, making them crucial in electronics and other technologies.
Examples of metalloids include boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), and polonium (Po).
Contrasting Noble Gases and Metalloids: A Clear Distinction
The fundamental differences between noble gases and metalloids are stark:
| Feature | Noble Gases | Metalloids |
|---|---|---|
| Reactivity | Extremely low (inert) | Intermediate |
| Electron Configuration | Full valence shell (8 electrons, except He) | Incompletely filled valence shell |
| Electrical Conductivity | Poor | Semiconductor (variable conductivity) |
| Physical State at Room Temperature | Gases | Solids |
| Applications | Lighting, cryogenics, welding, etc. | Electronics, semiconductors, alloys, etc. |
The contrasting properties listed above clearly demonstrate the vast differences between these two groups. The crucial distinction lies in their electron configurations and the resulting reactivity. Noble gases, with their complete valence shells, are extremely unreactive, while metalloids, with incomplete valence shells, possess intermediate reactivity and semiconducting properties. These differences are so significant that classifying a metalloid as a noble gas would be fundamentally inaccurate.
The Importance of Accurate Classification in Chemistry
The precise classification of elements is paramount in chemistry. This accurate categorization allows chemists to:
- Predict Chemical Behavior: Knowing the group an element belongs to helps predict its reactivity and the types of compounds it may form.
- Design and Synthesize New Materials: Understanding the properties of elements enables the development of novel materials with specific characteristics.
- Develop Technological Applications: The unique properties of elements are exploited in various technological advancements.
Misclassifying elements, such as labeling a metalloid as a noble gas, would lead to inaccurate predictions of their chemical behavior and hinder the development of effective applications.
Exploring Misconceptions and Addressing Common Queries
The clear distinction between metalloids and noble gases sometimes gets muddled, leading to common misconceptions. Let's address some frequent questions:
-
"Metalloids sometimes exhibit metallic luster, so aren't they a bit like noble gases?" Metallic luster is a physical property, not a chemical one. Noble gases are chemically inert; their luster (or lack thereof) is irrelevant to their chemical classification.
-
"Both metalloids and noble gases exist in different states at room temperature; doesn't this suggest some similarity?" While true that metalloids are typically solid while noble gases are gaseous at room temperature, this difference in physical state is a result of their vastly different interatomic forces and not indicative of a chemical similarity.
-
"Aren't there exceptions to the rule? Some noble gases form compounds, just like some metalloids." The formation of compounds by certain noble gases under extraordinary conditions does not negate the fundamental difference in their overall reactivity and electron configuration compared to metalloids. These exceptions are rare and do not invalidate the general rule of their inertness.
Conclusion: A Firm Distinction Remains
In conclusion, metalloids are definitively not noble gases. Their properties, stemming from their electron configurations and chemical behavior, are fundamentally different. Metalloids are semiconductors with intermediate reactivity, while noble gases are exceptionally inert due to their complete valence shells. Understanding this clear distinction is crucial for accurate chemical prediction, material design, and technological advancements. The periodic table's organization provides a powerful framework for understanding the diverse properties of elements, emphasizing the importance of careful categorization and the avoidance of misconceptions. The persistent differences in reactivity, electron configurations, and general behavior firmly establish the separate identities of metalloids and noble gases, ensuring that their distinct characteristics are properly recognized and utilized in various scientific and technological endeavors.
Latest Posts
Latest Posts
-
How Many Seconds In A Year Scientific Notation
Mar 29, 2025
-
How Many Chiral Centers Are Present In The Following Structure
Mar 29, 2025
-
What Is The Velocity In M Seconds Of Nerves Impules
Mar 29, 2025
-
Which Of The Following Is Not A Capital Expenditure
Mar 29, 2025
-
How Long Does It Take To Read 120 Pages
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is A Metaloids Considered A Noble Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
