How Many Chiral Centers Are Present In The Following Structure
News Leon
Mar 29, 2025 · 5 min read
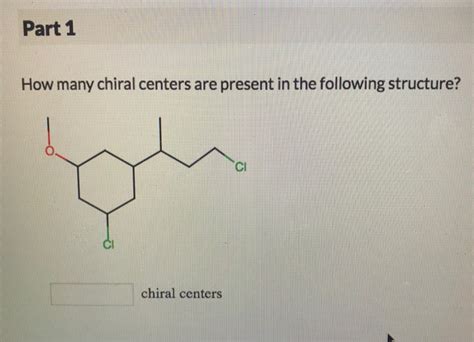
Table of Contents
How Many Chiral Centers Are Present in a Given Structure? A Comprehensive Guide
Determining the number of chiral centers in a molecule is a fundamental concept in organic chemistry. Chiral centers, also known as stereocenters, are carbon atoms bonded to four different groups. The presence of chiral centers leads to stereoisomerism, significantly impacting a molecule's properties and biological activity. This comprehensive guide will walk you through the process of identifying chiral centers, explain the importance of this determination, and delve into advanced considerations for complex structures.
Understanding Chiral Centers and Stereocenters
Before we dive into counting chiral centers, let's solidify our understanding of the fundamental concept. A chiral center (or stereocenter) is a carbon atom that is sp<sup>3</sup> hybridized and bonded to four different substituents. This asymmetry leads to the molecule existing in two non-superimposable mirror images, called enantiomers. These enantiomers have identical physical properties (except for their interaction with plane-polarized light) but can exhibit vastly different biological activities.
Key Characteristics of a Chiral Center:
- Sp<sup>3</sup> Hybridization: The carbon atom must be tetrahedral, meaning it has four single bonds.
- Four Different Substituents: Each of the four bonds must connect to a different atom or group of atoms. If two or more substituents are identical, the carbon is not a chiral center.
Identifying Chiral Centers: A Step-by-Step Approach
Let's outline a methodical approach to identifying chiral centers in any given molecular structure:
-
Identify all sp<sup>3</sup> hybridized carbon atoms: Begin by carefully examining the structure and locating all carbon atoms with four single bonds. These are the potential chiral centers.
-
Assess the substituents on each sp<sup>3</sup> carbon: For each potential chiral center, analyze the four groups or atoms attached to it. Determine if all four groups are unique.
-
Look for identical substituents: If any two or more substituents are identical (even if they are part of larger groups), that carbon atom is not a chiral center. The molecule will not exhibit chirality at that point.
-
Count the chiral centers: Once you have systematically evaluated each sp<sup>3</sup> carbon, count the number of carbons that meet the criteria of having four unique substituents. This number represents the total number of chiral centers in the molecule.
Illustrative Examples: Simple and Complex Structures
Let's illustrate the process with examples, starting with simpler structures and progressing to more complex ones. Remember, we'll focus on the methodology rather than providing specific structures visually. The principle remains the same, regardless of how the structure is presented.
Example 1: A Simple Structure
Imagine a molecule with a central carbon atom bonded to a methyl group (CH<sub>3</sub>), a hydroxyl group (OH), a hydrogen atom (H), and a chlorine atom (Cl). In this case, all four substituents are different. Therefore, this central carbon is a chiral center. The molecule possesses one chiral center.
Example 2: A More Complex Structure
Consider a molecule containing a chain of carbons. Some carbons might have two identical substituents (e.g., two methyl groups), while others might have four distinct substituents. Systematically examine each sp<sup>3</sup> carbon. Any carbon with two or more identical substituents is not a chiral center. The number of chiral centers is the count of those carbons that pass the 'four unique substituents' test.
Example 3: Cyclic Structures
Cyclic structures (rings) present no special difficulties. Follow the same procedure. Examine each sp<sup>3</sup> carbon atom in the ring. Consider each substituent connected to it—whether it is another carbon in the ring or a side group. If all four are different, it's a chiral center.
Example 4: Structures with Internal Symmetry
Sometimes, a molecule might possess internal symmetry. This symmetry can lead to some carbons appearing to have four different substituents but actually don't due to the overall symmetry of the molecule. Careful examination is crucial in these cases to avoid overcounting chiral centers.
Example 5: Dealing with Double Bonds
Remember, a chiral center must have four single bonds. Carbon atoms involved in double or triple bonds cannot be chiral centers. Focus your attention solely on sp<sup>3</sup> hybridized carbons.
Advanced Considerations: Meso Compounds and Pseudoasymmetry
While the basic principles are straightforward, certain scenarios warrant a more in-depth understanding.
Meso Compounds:
Meso compounds are molecules that possess chiral centers but are nonetheless achiral. This arises from an internal plane of symmetry that makes the molecule superimposable on its mirror image. Even though they contain chiral centers, meso compounds are not optically active. Identifying meso compounds requires careful analysis of the molecular symmetry.
Pseudoasymmetry:
Pseudoasymmetric centers are different from typical chiral centers. They involve a carbon atom with four different groups, but two of these groups are enantiomers of each other. This unique situation still leads to stereoisomerism, but the analysis becomes more nuanced.
The Importance of Identifying Chiral Centers
The ability to identify chiral centers is critical for various reasons:
-
Predicting the number of stereoisomers: A molecule with 'n' chiral centers can have up to 2<sup>n</sup> stereoisomers (excluding meso compounds).
-
Understanding drug activity: Enantiomers often exhibit drastically different biological activities. One enantiomer might be highly effective, while the other could be inactive or even toxic. Knowing the chiral centers is vital in pharmaceutical development and drug design.
-
Designing and interpreting chemical reactions: Chiral centers influence the stereochemical outcome of chemical reactions. Understanding the stereochemistry is crucial for controlling reaction pathways and obtaining desired products.
-
Analyzing spectroscopic data: Chiral centers affect the spectroscopic properties (NMR, etc.) of molecules, providing valuable clues for structure elucidation.
Conclusion: A Practical Skill for Chemists
Determining the number of chiral centers in a molecule is an essential skill for any organic chemist. The steps outlined above provide a systematic approach for identifying these critical stereocenters. While relatively straightforward for simple structures, a thorough understanding is necessary for tackling more complex molecules and recognizing situations like meso compounds and pseudoasymmetry. Remember, precise identification of chiral centers is crucial for understanding molecular properties, predicting reaction outcomes, and advancing our knowledge in various chemical fields. This comprehensive guide serves as a valuable resource for mastering this fundamental concept in organic chemistry.
Latest Posts
Latest Posts
-
One Billion Equal To How Many Crores
Mar 31, 2025
-
If The Cross Product Of Two Vectors Is Zero
Mar 31, 2025
-
How To Use Insert In Python
Mar 31, 2025
-
How Many Lines Of Symmetry Does Scalene Triangle Have
Mar 31, 2025
-
What Is The Ultimate Purpose Of A Political Party
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Chiral Centers Are Present In The Following Structure . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
