Is A Base A Proton Acceptor
News Leon
Apr 01, 2025 · 6 min read
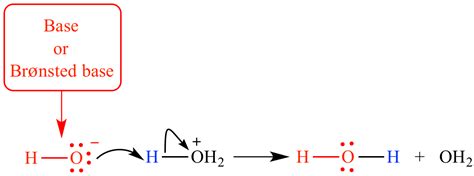
Table of Contents
Is a Base a Proton Acceptor? A Deep Dive into Acid-Base Chemistry
The seemingly simple question, "Is a base a proton acceptor?" opens the door to a fascinating world of acid-base chemistry. While the answer is a resounding yes, according to the Brønsted-Lowry definition, the nuances and implications of this definition are far richer than a simple yes or no. This article will delve deep into the concept, exploring various acid-base theories, examining the role of protons in chemical reactions, and clarifying common misconceptions.
Understanding the Brønsted-Lowry Definition
The most widely accepted definition of acids and bases is the Brønsted-Lowry theory. This theory defines an acid as a proton donor and a base as a proton acceptor. A proton, in this context, refers to a hydrogen ion (H⁺), which is simply a hydrogen atom that has lost its electron.
The key to understanding this definition lies in the transfer of protons. When an acid reacts with a base, the acid donates a proton to the base. This proton transfer is the defining characteristic of a Brønsted-Lowry acid-base reaction. For example, consider the reaction between hydrochloric acid (HCl) and water (H₂O):
HCl + H₂O → H₃O⁺ + Cl⁻
In this reaction, HCl acts as the acid, donating a proton to H₂O. Water, in turn, acts as the base, accepting the proton to form the hydronium ion (H₃O⁺). This illustrates the core principle of the Brønsted-Lowry theory: acid-base reactions involve the transfer of a proton from an acid to a base.
Conjugate Acid-Base Pairs
An important concept related to the Brønsted-Lowry theory is that of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. Similarly, when a base accepts a proton, it forms its conjugate acid. In the HCl and H₂O reaction above:
- HCl (acid) donates a proton to become Cl⁻ (conjugate base)
- H₂O (base) accepts a proton to become H₃O⁺ (conjugate acid)
Understanding conjugate pairs is crucial for predicting the outcome of acid-base reactions and analyzing their equilibrium.
Beyond Brønsted-Lowry: Other Acid-Base Theories
While the Brønsted-Lowry theory is widely used, it's not the only way to define acids and bases. Other important theories include:
Arrhenius Theory
The Arrhenius theory, a precursor to the Brønsted-Lowry theory, defines acids as substances that produce hydrogen ions (H⁺) in aqueous solution and bases as substances that produce hydroxide ions (OH⁻) in aqueous solution. While simpler, this theory is limited as it only applies to aqueous solutions and doesn't encompass all acid-base reactions.
Lewis Theory
The Lewis theory provides a broader definition of acids and bases. A Lewis acid is defined as an electron-pair acceptor, and a Lewis base is defined as an electron-pair donor. This theory expands the scope of acid-base chemistry to include reactions that don't involve proton transfer. For example, the reaction between boron trifluoride (BF₃) and ammonia (NH₃) is considered a Lewis acid-base reaction:
BF₃ + NH₃ → F₃B-NH₃
In this reaction, BF₃ acts as a Lewis acid, accepting an electron pair from NH₃, which acts as a Lewis base. Note that no proton transfer occurs.
The Importance of Proton Transfer
The emphasis on proton transfer in the Brønsted-Lowry definition highlights the importance of the hydrogen ion's unique properties. The small size and high charge density of the proton make it highly reactive. Its transfer between molecules significantly alters their properties, influencing factors like pH, solubility, and reactivity.
Proton transfer reactions are fundamental to many biological processes, including enzyme catalysis, protein folding, and DNA replication. Understanding these reactions is crucial in various fields, including medicine, biochemistry, and environmental science.
Common Misconceptions about Bases and Proton Acceptance
Despite the seemingly straightforward definition, some common misconceptions surround bases and their proton-accepting nature:
Misconception 1: All bases contain hydroxide ions (OH⁻).
This is incorrect, especially outside the scope of the Arrhenius theory. Many bases, like ammonia (NH₃), do not contain hydroxide ions but can still accept protons.
Misconception 2: Proton acceptance always leads to a significant pH change.
While proton acceptance often leads to a pH increase (making the solution more basic), the magnitude of the change depends on the strength of the base and the concentration of the solution. Weak bases may only cause a small pH change.
Misconception 3: Only molecules can act as bases.
Ions can also act as bases. For instance, the carbonate ion (CO₃²⁻) is a common base that readily accepts protons.
Misconception 4: Proton acceptance is always a fast reaction.
The speed of proton transfer reactions varies widely, depending on factors like the strength of the acid and base, temperature, and solvent. Some reactions are extremely fast, while others are slow.
Strength of Bases and Proton Affinity
The ability of a base to accept a proton is related to its strength. Strong bases readily accept protons, while weak bases accept protons less readily. The strength of a base is often quantified by its proton affinity, which is the energy released when a base accepts a proton. Higher proton affinity indicates a stronger base.
Factors influencing base strength include:
- Electronegativity: Bases with more electronegative atoms are generally weaker because they hold onto their electron pairs more tightly, making it harder for them to accept a proton.
- Size and charge: Larger and more negatively charged bases tend to be stronger because they can better stabilize the negative charge that results from proton acceptance.
- Resonance: Bases with resonance structures can better delocalize the negative charge resulting from protonation, making them stronger.
Applications of Proton Acceptor Bases
The ability of bases to accept protons underpins their widespread use in numerous applications, including:
- Neutralization reactions: Bases are used to neutralize acids, forming salts and water. This is crucial in various industrial processes and environmental remediation.
- pH control: Bases are essential for controlling pH in various applications, such as in chemical synthesis, food processing, and wastewater treatment.
- Catalysis: Many bases act as catalysts in chemical reactions, facilitating the proton transfer steps that are necessary for the reaction to proceed.
- Buffer solutions: Mixtures of weak acids and their conjugate bases (or weak bases and their conjugate acids) are used to create buffer solutions, which resist changes in pH.
Conclusion
In conclusion, the answer to the question "Is a base a proton acceptor?" is definitively yes, according to the widely accepted Brønsted-Lowry definition. This seemingly simple concept unveils a complex and fascinating aspect of chemistry, impacting various fields of study and application. Understanding the nuances of proton transfer, conjugate acid-base pairs, and the different acid-base theories provides a deeper appreciation for the role of bases in chemical reactions and their significance in various aspects of the world around us. From neutralizing acidic spills to catalyzing essential biological processes, the ability of bases to accept protons is a fundamental principle driving a vast array of chemical phenomena. Further exploration of this concept opens avenues to advanced topics like acid-base equilibrium, titration, and the intricate mechanisms of chemical reactions.
Latest Posts
Latest Posts
-
What Is The Oxidation Number Of Sulfur In Na2s2o3
Apr 02, 2025
-
Select The Correct Statement About Osmosis
Apr 02, 2025
-
Does Reactivity Increase Down A Group
Apr 02, 2025
-
How Many Vertices Does Octagon Have
Apr 02, 2025
-
Reaction Of Ammonia And Sulfuric Acid
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Is A Base A Proton Acceptor . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
