How Many Valence Electrons Does Gallium Have
News Leon
Mar 24, 2025 · 5 min read
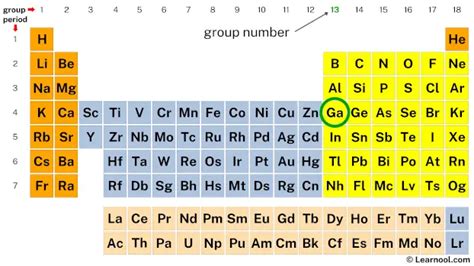
Table of Contents
How Many Valence Electrons Does Gallium Have? A Deep Dive into Gallium's Electronic Structure
Gallium, a fascinating element with a wide array of applications, sits nestled in the periodic table's 13th group (or IIIA group using the older numbering system). Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and the properties that make it so useful. This comprehensive guide will explore the answer to the question "How many valence electrons does gallium have?" and delve deeper into the implications of this electronic configuration.
Understanding Valence Electrons
Before we pinpoint gallium's valence electrons, let's clarify what valence electrons are. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the most loosely held and, therefore, most likely to participate in chemical bonding. They determine an element's reactivity, its ability to form chemical bonds with other atoms, and the types of bonds it can form (ionic, covalent, metallic, etc.). The number of valence electrons significantly influences the element's chemical properties and its position in the periodic table.
Determining Gallium's Valence Electrons
Gallium (Ga) has an atomic number of 31, meaning it has 31 protons and, in its neutral state, 31 electrons. To determine its valence electrons, we need to understand its electronic configuration. This configuration describes how the electrons are distributed among the different energy levels and subshells within the atom.
The electronic configuration of gallium is 1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p¹.
Let's break this down:
-
1s², 2s², 2p⁶, 3s², 3p⁶, 3d¹⁰: These represent the inner shells, which are completely filled. The electrons in these shells are tightly bound to the nucleus and don't usually participate in chemical bonding. They are called core electrons.
-
4s²4p¹: This is the outermost shell, the valence shell. It contains three electrons: two in the 4s subshell and one in the 4p subshell.
Therefore, gallium has three valence electrons.
The Significance of Gallium's Three Valence Electrons
This seemingly simple number – three – dictates a wealth of gallium's properties and its chemical behavior. Let's explore some key consequences:
1. Chemical Reactivity:
Gallium's three valence electrons make it moderately reactive. It's not as reactive as the alkali metals (Group 1) with only one valence electron, nor is it as unreactive as the noble gases (Group 18) with a full valence shell. This moderate reactivity allows for a diverse range of chemical interactions.
2. Bonding Behavior:
Gallium primarily forms covalent bonds, though it can also exhibit metallic bonding. The three valence electrons are available to share with other atoms to achieve a more stable electronic configuration. The formation of covalent bonds is crucial in many of gallium's compounds, particularly its semiconductors and organometallic compounds.
3. Oxidation States:
The three valence electrons allow gallium to exhibit several oxidation states, primarily +1 and +3. The +3 oxidation state is more common because losing three electrons leads to a stable, filled 3d subshell. However, the +1 oxidation state is also observed, especially in compounds where steric hindrance or other factors stabilize the univalent state. This versatility in oxidation states further contributes to the chemical diversity of gallium compounds.
4. Semiconductor Properties:
Gallium's electronic structure is a key reason for its importance in semiconductor technology. Specifically, gallium arsenide (GaAs) is a prominent compound semiconductor. The bonding arrangement between gallium and arsenic results in a band gap that allows for efficient electrical conductivity control, making GaAs an essential component in high-speed electronic devices and optoelectronic applications like lasers and LEDs. The three valence electrons from gallium play a vital role in achieving the appropriate electronic structure in these materials.
Gallium's Applications: A Consequence of its Electronic Structure
The unique properties stemming from gallium's three valence electrons have led to its extensive use in various technological applications:
1. Semiconductor Industry:
- Gallium arsenide (GaAs): Used in high-speed integrated circuits, microwave devices, and optoelectronic devices (lasers, LEDs). The tunable band gap allows for tailoring these devices to specific applications.
- Gallium nitride (GaN): Used in high-efficiency power electronics, LEDs for high-brightness displays, and UV detectors. Its wide band gap allows for operation at higher voltages and temperatures than silicon-based devices.
- Indium gallium nitride (InGaN): A versatile semiconductor alloy used in various applications, including white LEDs and laser diodes.
2. Medical Applications:
- Radiopharmaceuticals: Gallium-67 is used in nuclear medicine for imaging tumors and detecting infections. Its chemical properties allow it to concentrate in specific tissues.
- Cancer Treatment: Gallium compounds are under investigation as potential anticancer agents.
3. Other Applications:
- Alloys: Gallium is added to other metals to improve their properties, such as lower melting points or enhanced corrosion resistance. Galinstan, an alloy of gallium, indium, and tin, is used as a mercury-free substitute in thermometers and other applications.
- Mirrors: Gallium can be used to coat mirrors due to its high reflectivity.
- Optical fibers: Gallium compounds are involved in the manufacture of specialized optical fibers.
Comparing Gallium to Other Group 13 Elements
Gallium's behavior can be understood by comparing it to other elements in Group 13 (Boron, Aluminum, Indium, Thallium). While all share three valence electrons, their properties vary due to differences in size, electronegativity, and relativistic effects (particularly prominent in heavier elements).
- Boron: A metalloid with a significantly smaller size, boron forms predominantly covalent bonds and is less metallic than gallium.
- Aluminum: More reactive than gallium, aluminum is widely used in various applications due to its abundance and lightness.
- Indium: Similar to gallium in terms of reactivity and applications in semiconductors.
- Thallium: More toxic than the lighter elements in the group, thallium exhibits more pronounced relativistic effects influencing its properties.
Conclusion: The Importance of Valence Electrons in Understanding Gallium
The simple answer is that gallium has three valence electrons. However, this seemingly straightforward fact is the cornerstone of understanding gallium's rich chemistry and its diverse applications in modern technology and medicine. The number of valence electrons dictates its reactivity, bonding behavior, oxidation states, and ultimately, the remarkable properties that have made gallium an essential element in various fields. From semiconductors to medicine, the influence of these three electrons extends far beyond a simple numerical value. The deep understanding of gallium's electronic structure is vital for continued advancements in material science and technological innovations.
Latest Posts
Latest Posts
-
How Many Valence Electrons Does As Have
Mar 26, 2025
-
Select The Correct Statement About Cardiac Output
Mar 26, 2025
-
Why Is Dna Replication Called Semiconservative
Mar 26, 2025
-
To Whom Did India Give The Title Mahatma
Mar 26, 2025
-
The Temperature At Which A Solid Becomes A Liquid
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Gallium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
