How Many Valence Electrons Does As Have
News Leon
Mar 26, 2025 · 5 min read
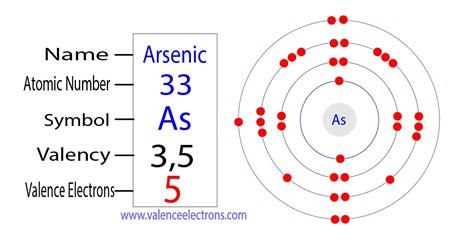
Table of Contents
How Many Valence Electrons Does Arsenic (As) Have? Understanding Arsenic's Electronic Structure
Arsenic (As), a metalloid element residing in Group 15 (or VA) of the periodic table, plays a fascinating role in both the natural world and various technological applications. Understanding its chemical behavior necessitates a thorough grasp of its electronic structure, particularly the number of valence electrons it possesses. This article delves into the intricacies of arsenic's electron configuration, explaining not only how many valence electrons it has but also the implications of this number for its chemical bonding and reactivity.
Delving into Atomic Structure: Electrons, Shells, and Subshells
Before we determine the number of valence electrons in arsenic, let's refresh our understanding of atomic structure. Atoms consist of a nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons are arranged in distinct energy levels or shells, each capable of holding a specific maximum number of electrons. Further, each shell is subdivided into subshells (s, p, d, and f), each with its unique electron capacity.
The arrangement of electrons in an atom is described by its electron configuration, a shorthand notation indicating the distribution of electrons across shells and subshells. This configuration dictates an atom's chemical properties, determining how it interacts with other atoms to form chemical bonds. The outermost shell's electrons, the valence electrons, are the primary players in chemical bonding.
Arsenic's Electron Configuration and Valence Electrons
Arsenic has an atomic number of 33, meaning it possesses 33 protons and 33 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle, filling the lowest energy levels first. The electron configuration of arsenic is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p³
Let's break this down:
- 1s²: The first shell (n=1) contains one s subshell, holding a maximum of two electrons.
- 2s² 2p⁶: The second shell (n=2) contains one s subshell (2 electrons) and one p subshell (6 electrons).
- 3s² 3p⁶: The third shell (n=3) also contains an s subshell (2 electrons) and a p subshell (6 electrons).
- 4s² 3d¹⁰: The fourth shell (n=4) begins with an s subshell (2 electrons). Note that the 3d subshell, despite being in the third shell, has a slightly higher energy level than the 4s subshell, and is therefore filled next. It holds a maximum of 10 electrons.
- 4p³: Finally, the fourth shell's p subshell holds three electrons.
The valence electrons are the electrons in the outermost shell, which, in arsenic's case, is the fourth shell (n=4). These are the 4s² and 4p³ electrons.
Therefore, arsenic has a total of five valence electrons (2 + 3 = 5).
Implications of Arsenic's Five Valence Electrons
The presence of five valence electrons significantly influences arsenic's chemical behavior and reactivity:
-
Chemical Bonding: Arsenic's five valence electrons allow it to form a variety of chemical bonds. It can gain three electrons to achieve a stable octet (eight electrons in its outermost shell), forming arsenide anions (As³⁻). Alternatively, it can share its five valence electrons through covalent bonding, forming three single bonds and one lone pair. This ability to form multiple bonds leads to arsenic's existence in various compounds with diverse structures and properties.
-
Oxidation States: Arsenic exhibits multiple oxidation states, reflecting its capacity to either lose or gain electrons. Common oxidation states include -3, +3, and +5, which correspond to gaining three electrons, losing three electrons, or losing five electrons, respectively. The variability in oxidation states contributes to arsenic's diverse chemistry.
-
Allotropes: Arsenic exists in several allotropic forms, meaning it can exist in different structural modifications. The most common allotrope is gray arsenic, a crystalline solid with metallic properties. The varied bonding arrangements in different allotropes are directly related to its valence electron configuration.
Arsenic in the Environment and Its Toxicity
Arsenic is a naturally occurring element found in various minerals and rocks. It can be released into the environment through natural processes like volcanic eruptions or weathering, as well as through human activities such as mining and industrial processes. Arsenic contamination of water sources is a significant environmental concern, posing significant health risks.
The toxicity of arsenic stems from its ability to interfere with various biochemical processes. Arsenic compounds can bind to crucial enzyme sites, inhibiting their function and disrupting cellular metabolism. Long-term exposure to arsenic can lead to various health problems, including skin lesions, cardiovascular disease, and cancer.
Arsenic's Applications in Technology and Industry
Despite its toxicity, arsenic finds applications in various technological and industrial fields:
-
Semiconductors: Arsenic is used in the production of semiconductors, especially gallium arsenide (GaAs), which exhibits superior electronic properties compared to silicon in certain applications. GaAs is used in high-speed electronic devices, lasers, and solar cells.
-
Alloys: Arsenic is added to certain alloys to improve their properties, such as hardness and corrosion resistance.
-
Pesticides: Although its use is declining due to its toxicity, arsenic compounds were historically used as insecticides and pesticides.
Conclusion: The Significance of Valence Electrons in Arsenic's Chemistry
The presence of five valence electrons in arsenic fundamentally determines its chemical behavior and reactivity. This relatively high number of valence electrons allows arsenic to form various bonds, exhibit multiple oxidation states, and exist in different allotropic forms. Understanding arsenic's electronic structure is crucial not only for comprehending its diverse chemical properties but also for addressing environmental concerns related to arsenic contamination and utilizing its unique properties in technological applications. Further research continues to expand our knowledge of arsenic's chemistry and its implications for human health and the environment. The seemingly simple question of "How many valence electrons does arsenic have?" opens a door to a complex and fascinating world of chemical interactions and their consequences. From the subtle shifts in its electron configuration to the significant impact on human health and technological advancements, arsenic’s five valence electrons tell a compelling story of chemical diversity and environmental consequence.
Latest Posts
Latest Posts
-
A Long Nonconducting Solid Cylinder Of Radius
Mar 29, 2025
-
What Will Happen If Ribosomes Are Removed From The Cell
Mar 29, 2025
-
How Many Hydrogen Bonds Between C And G
Mar 29, 2025
-
Regulates What Enters And Leaves The Cell
Mar 29, 2025
-
The Most Active Phagocytic Cells In Circulating Blood Are
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does As Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
