How Many Hydrogen Bonds Between C And G
News Leon
Mar 29, 2025 · 6 min read
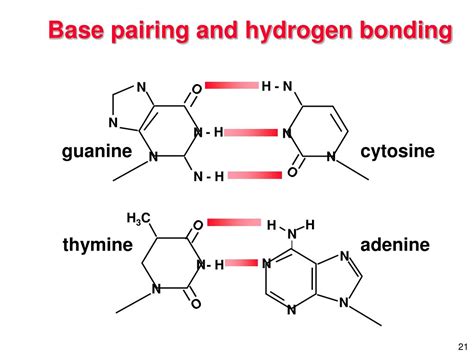
Table of Contents
How Many Hydrogen Bonds Between C and G? Decoding the Base Pairing in DNA
Understanding the intricacies of DNA's double helix structure is fundamental to comprehending the mechanisms of life. At the heart of this structure lies the specific pairing of nitrogenous bases: adenine (A) with thymine (T), and cytosine (C) with guanine (G). While the A-T pair is relatively straightforward, the C-G pairing presents a slightly more complex picture due to the number of hydrogen bonds involved. This article will delve deep into the specifics of C-G base pairing, exploring the number of hydrogen bonds, the significance of this number, and its implications for DNA stability and function.
The Fundamentals of Base Pairing: Hydrogen Bonds as the Glue
The double helix structure of DNA is maintained by hydrogen bonds forming between complementary bases on opposite strands. These bonds are relatively weak individually but, collectively, they provide the necessary stability for the DNA molecule to maintain its structure and perform its crucial functions. Hydrogen bonds are electrostatic attractions between a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen or nitrogen) and another electronegative atom. This type of bond is weaker than a covalent bond but plays a crucial role in the structural integrity and functionality of biological macromolecules, including DNA.
Why Hydrogen Bonds are Crucial in DNA
The choice of hydrogen bonding in DNA is not arbitrary. The strength of these bonds is precisely what's needed. They are strong enough to hold the two strands together under normal cellular conditions, yet weak enough to allow the strands to separate during processes like DNA replication and transcription. If the bonds were too strong, separating the strands would require excessive energy and potentially damage the DNA. If they were too weak, the double helix would constantly unravel, rendering it unstable and non-functional.
The C-G Base Pair: A Tale of Three Hydrogen Bonds
Unlike the A-T base pair, which is held together by two hydrogen bonds, the C-G base pair is characterized by three hydrogen bonds. This seemingly small difference in the number of hydrogen bonds has significant consequences for the stability and properties of the DNA molecule. Let's examine these bonds in detail:
- Bond 1: A hydrogen bond forms between the amino group (-NH2) of cytosine and the carbonyl group (=O) of guanine.
- Bond 2: A hydrogen bond forms between the N-H group of cytosine and the nitrogen atom (N) of guanine.
- Bond 3: A hydrogen bond forms between the amino group (-NH2) of guanine and the nitrogen atom (N) of cytosine.
These three hydrogen bonds contribute to a stronger interaction between C and G compared to the A-T pair. This stronger interaction has implications for several aspects of DNA's behavior.
Visualizing the Three Hydrogen Bonds
It's helpful to visualize the three hydrogen bonds between C and G. Numerous scientific illustrations and models depict the precise arrangement and geometry of these bonds, emphasizing the spatial complementarity of the bases. These visualizations often highlight the precise positioning of the hydrogen bond donors (atoms with hydrogen attached) and acceptors (electronegative atoms). The optimal geometry ensures maximum strength and stability of the interaction.
The Significance of the Three Hydrogen Bonds: Stability and Melting Temperature
The presence of three hydrogen bonds in the C-G base pair leads to a higher thermal stability compared to the A-T base pair. This increased stability is reflected in the melting temperature (Tm) of DNA. The melting temperature represents the temperature at which half of the DNA double helix denatures (separates into single strands). DNA with a higher GC content (a greater proportion of C-G base pairs) will have a higher Tm than DNA with a higher AT content.
GC Content and DNA Stability
The higher Tm of GC-rich DNA reflects the cumulative effect of the additional hydrogen bond in each C-G pair. The stronger interaction between C and G bases requires more energy to break these bonds compared to the weaker A-T interactions. This increased stability is crucial for DNA's function. In organisms that thrive in high-temperature environments, for example, a higher GC content is often observed in their DNA to maintain the stability of the genome under thermal stress.
Implications for DNA Replication and Transcription
The number of hydrogen bonds between bases also influences the speed and efficiency of DNA replication and transcription. The enzymes involved in these processes need to separate the DNA strands to access the individual bases. The stronger C-G bonds can create a slight "roadblock" for these enzymes, potentially slowing down the replication or transcription process in regions with high GC content. Conversely, regions with higher AT content tend to unwind more easily, facilitating faster replication and transcription.
Impact on Gene Expression
The differences in base pairing stability can even have subtle effects on gene expression. The DNA regions that control gene expression (promoters and enhancers) often have specific GC or AT content patterns. The stability of these regions might influence the accessibility of the DNA to the regulatory proteins, thus affecting the rate of gene transcription.
Beyond the Basics: Factors Influencing Hydrogen Bond Strength
While the number of hydrogen bonds is a primary determinant of base pair stability, other factors can also influence the strength of the interaction. These include:
- Solvent effects: The surrounding water molecules can affect the hydrogen bonding by competing for hydrogen bond donors and acceptors.
- Base stacking interactions: In addition to hydrogen bonds, the stacking interactions between adjacent base pairs contribute to the overall stability of the DNA double helix. These hydrophobic interactions are significant and add to the overall stability.
- Ionic strength: The presence of ions in the solution can also affect the strength of hydrogen bonds by shielding electrostatic charges.
- DNA sequence context: The surrounding base pairs can influence the local stability of a specific base pair.
Conclusion: The Significance of C-G Base Pairing
The fact that there are three hydrogen bonds between C and G, compared to two in the A-T pair, is a fundamental aspect of DNA's structure and function. This seemingly small difference has significant implications for the stability, melting temperature, and even the rate of replication and transcription. Understanding the nuances of C-G base pairing is crucial for appreciating the intricate mechanisms that govern the storage, replication, and expression of genetic information. Further research continues to explore the subtle complexities of these interactions and their role in various biological processes, reinforcing the critical role of these seemingly simple hydrogen bonds in the grand scheme of life. The precise number of hydrogen bonds, coupled with other contributing factors, underpins the remarkable stability and functionality of the DNA double helix – a molecule at the very core of life itself. This intricate interplay of forces underscores the elegance and efficiency of nature's design.
Latest Posts
Latest Posts
-
25 Is 4 Of What Number
Mar 31, 2025
-
Is Sand And Water A Solution
Mar 31, 2025
-
Is Hcl A Compound Or Element
Mar 31, 2025
-
All Of The Following Are Characteristics Of Perfect Competition Except
Mar 31, 2025
-
Rna Plays An Important Role In What Biological Process
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Hydrogen Bonds Between C And G . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
