How Many Valence Electrons Does Antimony Have
News Leon
Mar 15, 2025 · 5 min read
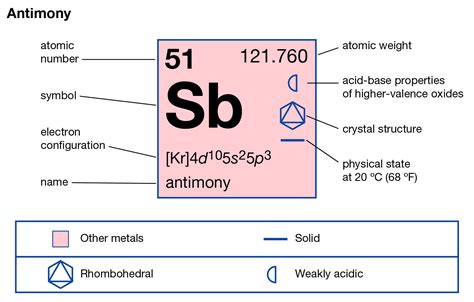
Table of Contents
How Many Valence Electrons Does Antimony Have? A Deep Dive into Antimony's Electronic Structure
Antimony (Sb), a metalloid residing in Group 15 (or VA) of the periodic table, is a fascinating element with unique properties bridging the gap between metals and nonmetals. Understanding its electronic structure, particularly the number of valence electrons, is crucial to comprehending its chemical behavior and diverse applications. So, how many valence electrons does antimony possess? The answer is five. But this seemingly simple answer opens a door to a wealth of information about antimony's reactivity, bonding characteristics, and its place within the larger context of the periodic table.
Understanding Valence Electrons: The Key to Reactivity
Before delving into antimony's specifics, let's establish a foundational understanding of valence electrons. These are the electrons located in the outermost shell of an atom. They are the primary participants in chemical bonding, determining an element's reactivity and the types of compounds it can form. The number of valence electrons directly influences an element's oxidation state and its tendency to gain, lose, or share electrons to achieve a stable electron configuration, often resembling that of a noble gas.
Antimony's Electronic Configuration: Unveiling the Valence Shell
Antimony's atomic number is 51, meaning it has 51 protons and, in its neutral state, 51 electrons. To determine the number of valence electrons, we need to examine its electron configuration. This configuration describes how electrons are distributed among the different energy levels and subshells within the atom. Antimony's electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p³
Notice the outermost shell, the fifth energy level (n=5). This shell contains the valence electrons. It comprises the 5s and 5p subshells, holding a total of five electrons (two in the 5s and three in the 5p subshell). These five electrons are readily available for chemical bonding, making antimony relatively reactive, especially in its higher oxidation states.
Antimony's Chemical Behavior: A Consequence of Five Valence Electrons
The presence of five valence electrons profoundly influences antimony's chemical behavior. Unlike elements with completely filled valence shells (noble gases), antimony is chemically reactive and seeks stability by interacting with other atoms. This interaction can take several forms:
1. Covalent Bonding: Sharing Electrons for Stability
Antimony can achieve a stable octet (eight electrons in its outermost shell) by sharing its five valence electrons with other atoms through covalent bonding. This often leads to the formation of molecules like stibine (SbH₃), a highly toxic gas analogous to ammonia (NH₃). In stibine, antimony shares three electrons to form three single covalent bonds with hydrogen atoms.
2. Metallic Bonding: A Sea of Electrons
Antimony exhibits metallic properties, a characteristic partly attributed to its valence electrons. In solid antimony, valence electrons are delocalized, meaning they are not tightly bound to individual atoms but rather move freely throughout the metallic lattice. This "sea" of electrons contributes to antimony's electrical conductivity, although it's significantly lower than that of true metals.
3. Ionic Bonding: Gaining or Losing Electrons
While less common than covalent bonding, antimony can participate in ionic bonding under certain conditions. It can either lose three electrons to form Sb³⁺ (a less stable and less common state) or gain three electrons to form Sb³⁻ (a more likely outcome with highly electronegative elements). This ability to exhibit multiple oxidation states (III and V being the most common) reflects the versatility of its five valence electrons.
Oxidation States: A Manifestation of Valence Electron Involvement
The oxidation state of an element represents the apparent charge on an atom in a compound, assuming that all bonds are completely ionic. Antimony's most common oxidation states are +3 and +5, directly related to its five valence electrons. In the +3 oxidation state, antimony loses three electrons, while in the +5 oxidation state, it loses all five valence electrons. The formation of these different oxidation states highlights the flexibility of antimony's bonding capabilities.
Examples of Antimony in Different Oxidation States:
- +3 oxidation state: Antimony trioxide (Sb₂O₃), antimony trichloride (SbCl₃)
- +5 oxidation state: Antimony pentoxide (Sb₂O₅), antimony pentachloride (SbCl₅)
Applications of Antimony: Leveraging its Unique Properties
The unique properties stemming from antimony's five valence electrons translate into a range of applications across various industries:
-
Flame retardants: Antimony trioxide (Sb₂O₃) is widely used as a flame retardant in plastics, textiles, and other materials. Its presence interferes with the combustion process, slowing the spread of flames.
-
Alloys: Antimony is added to lead-based alloys to enhance their hardness and mechanical strength, improving their performance in applications like lead-acid batteries and solders.
-
Semiconductors: Certain antimony compounds exhibit semiconductor properties, finding applications in electronic devices and sensors.
-
Medicinal applications: Historically, antimony compounds have been used in medicine, although their toxicity limits their current use to a much smaller extent.
Antimony's Position in the Periodic Table: Trends and Relationships
Antimony's position in Group 15 of the periodic table allows for comparisons and contrasts with other group members like nitrogen (N), phosphorus (P), arsenic (As), and bismuth (Bi). While all members of this group possess five valence electrons, their properties vary significantly due to differences in atomic size, electronegativity, and metallic character. Antimony’s properties often lie intermediate between those of arsenic and bismuth, reflecting the gradual transition from metalloid to metallic behavior within the group.
Conclusion: Antimony's Five Valence Electrons – A Story of Versatility
In conclusion, antimony possesses five valence electrons, a defining characteristic that governs its chemical behavior and diverse applications. These electrons enable antimony to participate in various bonding types, exhibit multiple oxidation states, and contribute to its unique properties as a metalloid. Understanding the role of these valence electrons provides invaluable insight into antimony's reactivity, its position within the periodic table, and its importance in various industrial and technological applications. The seemingly simple number "five" opens a window to a complex and fascinating world of chemical behavior and material science. Further exploration into the intricacies of antimony's electronic structure and its chemical reactivity will continue to uncover new applications and expand our understanding of this multifaceted element.
Latest Posts
Latest Posts
-
Iodine Is Essential For The Synthesis Of
Mar 15, 2025
-
Is Osmosis High To Low Or Low To High
Mar 15, 2025
-
Concave Mirror And Convex Mirror Difference
Mar 15, 2025
-
Which Is Not A Cranial Bone Of The Skull
Mar 15, 2025
-
Mountain Range That Separates Europe And Asia
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Antimony Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
