How Many Valence Electrons Are In As
News Leon
Mar 20, 2025 · 6 min read
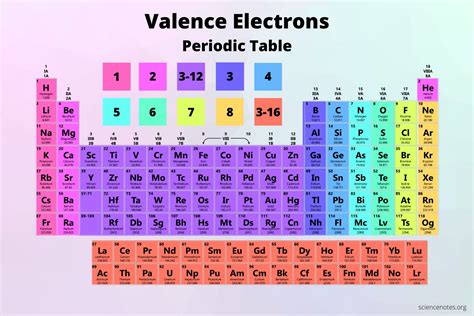
Table of Contents
How Many Valence Electrons Are in Arsenic (As)? Understanding Arsenic's Electronic Structure and Chemical Behavior
Arsenic (As), a metalloid element residing in Group 15 (or VA) of the periodic table, exhibits fascinating chemical properties largely dictated by its valence electrons. Understanding the number of valence electrons in arsenic is crucial to grasping its reactivity, bonding patterns, and overall behavior in various chemical contexts. This comprehensive article delves into the electronic structure of arsenic, explaining how to determine its valence electrons and exploring the implications of this number for its chemical characteristics.
Understanding Valence Electrons
Before focusing specifically on arsenic, let's establish a foundational understanding of valence electrons. Valence electrons are the outermost electrons in an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons is typically determined by the atom's position in the periodic table, specifically its group number.
Group Numbers and Valence Electrons
For main group elements (Groups 1-18), the group number (using the older numbering system) generally corresponds to the number of valence electrons. However, there are exceptions, particularly with transition metals and inner transition metals (lanthanides and actinides). For our purposes with arsenic, a main group element, this rule holds true.
Determining the Number of Valence Electrons in Arsenic
Arsenic (As) is located in Group 15 (or VA) of the periodic table. Based on the group number, arsenic possesses five valence electrons.
Electron Configuration and Valence Electrons
To understand this further, let's examine arsenic's electron configuration. The electron configuration describes the arrangement of electrons within an atom's electron shells and subshells. Arsenic's electron configuration is [Ar] 3d<sup>10</sup> 4s<sup>2</sup> 4p<sup>3</sup>.
- [Ar]: This represents the electron configuration of Argon, a noble gas, indicating that the inner shells are filled.
- 3d<sup>10</sup>: The 3d subshell is completely filled with 10 electrons. These electrons are inner electrons and do not participate in chemical bonding.
- 4s<sup>2</sup>: The 4s subshell contains two electrons.
- 4p<sup>3</sup>: The 4p subshell contains three electrons.
The valence electrons are the electrons in the outermost shell, which in arsenic's case is the fourth shell (n=4). This includes both the 4s and 4p electrons. Therefore, arsenic has a total of 2 (from 4s) + 3 (from 4p) = 5 valence electrons.
The Chemical Implications of Arsenic's Five Valence Electrons
The presence of five valence electrons significantly influences arsenic's chemical behavior. Let's explore some key implications:
1. Covalent Bonding:
Arsenic's five valence electrons allow it to form covalent bonds with other atoms. It can share these electrons to achieve a more stable electron configuration, often striving to gain a full octet (eight electrons) in its outermost shell. This explains why arsenic forms a wide range of covalent compounds with other nonmetals. For instance, arsenic can form As<sub>4</sub> molecules (similar to white phosphorus) where each arsenic atom forms three covalent bonds with other arsenic atoms. It also readily forms covalent bonds with halogens (fluorine, chlorine, bromine, iodine), oxygen, and hydrogen.
2. Oxidation States:
Arsenic exhibits a variety of oxidation states, reflecting its ability to gain or lose electrons. The most common oxidation states are +3 and +5. The +3 state occurs when arsenic loses three electrons, leaving two electrons in the 4s and a lone pair in the 4p orbital. The +5 oxidation state involves the loss of all five valence electrons, forming arsenic(V) compounds. The diverse oxidation states contribute to the wide range of arsenic-containing compounds with varying properties.
3. Allotropes:
Arsenic, like many other elements, exists in various allotropic forms. These allotropes have different structural arrangements and properties, arising from the different ways in which arsenic atoms bond to each other. The most common allotropic forms are yellow arsenic (molecular, As<sub>4</sub>) and gray arsenic (metallic), reflecting the versatility of bonding driven by the five valence electrons.
4. Semiconducting Properties:
Arsenic's semiconducting properties are a consequence of its electronic structure. The relatively small energy gap between its valence and conduction bands allows for the controlled movement of electrons, resulting in its utility in semiconductor materials and electronics. This controlled electron behavior is fundamentally linked to its number of valence electrons and their arrangement in its electronic structure.
5. Toxicity:
The toxicity of arsenic is linked to its chemical reactivity and the ability of its compounds to interfere with biological processes. The multiple oxidation states and varied bonding capabilities mean that arsenic can interact with crucial biomolecules, disrupting cellular functions and potentially leading to toxicity. Understanding the electronic structure, particularly the valence electrons, provides a framework for investigating the mechanistic details of arsenic toxicity.
Arsenic's Role in Different Fields
Arsenic, despite its toxicity, finds applications in several fields:
1. Metallurgy:
Arsenic is used as a doping agent in the semiconductor industry. Controlled addition of arsenic atoms to silicon or germanium crystals alters their electrical conductivity, forming n-type semiconductors vital in electronics. The precise number and behavior of arsenic's valence electrons are crucial in this process.
2. Pharmaceuticals:
Historically, arsenic compounds have been used in medicine, although their use is now restricted due to toxicity concerns. Some arsenic compounds have shown promise in treating certain cancers, highlighting the complex interplay between arsenic's toxicity and its potential therapeutic benefits. The research into these applications often focuses on the specific ways in which the five valence electrons contribute to the interactions between arsenic compounds and biological targets.
3. Agriculture:
Insecticides and herbicides historically contained arsenic compounds. However, due to their toxicity, their use is now significantly restricted in many regions. The use of these compounds, though now largely discontinued, demonstrates the influence of arsenic's chemical reactivity (related to its valence electrons) on its interaction with organisms.
Conclusion
The number of valence electrons in arsenic, definitively five, is a cornerstone to understanding its multifaceted chemical behavior. This number dictates its ability to form covalent bonds, exhibit varying oxidation states, exist in multiple allotropic forms, display semiconducting properties, and ultimately influence its toxicity and applications across various scientific and technological domains. By understanding arsenic's electronic structure and the role of its valence electrons, we gain a deeper appreciation for its complex chemistry and its implications in diverse fields. Further research into arsenic's properties continues to uncover new applications and insights into its behavior. This knowledge is vital for safe handling, responsible utilization, and the continued development of arsenic-based technologies and treatments.
Latest Posts
Latest Posts
-
Which Of The Following Combinations Are Correctly Matched
Mar 28, 2025
-
The Figure Gives An Overhead View Of The Path
Mar 28, 2025
-
A Galvanometer Has A Resistance Of 20 Ohm
Mar 28, 2025
-
What Are The Coordinates Of Point Q
Mar 28, 2025
-
30 As A Product Of Prime Factors
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
