How Many Sigma And Pi Bonds In A Triple Bond
News Leon
Mar 17, 2025 · 5 min read
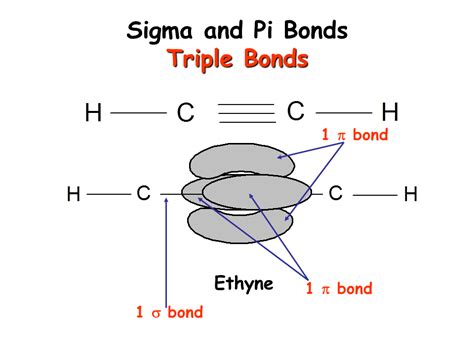
Table of Contents
- How Many Sigma And Pi Bonds In A Triple Bond
- Table of Contents
- How Many Sigma and Pi Bonds in a Triple Bond? A Deep Dive into Chemical Bonding
- The Fundamentals: Sigma and Pi Bonds
- Sigma (σ) Bonds: The Foundation
- Pi (π) Bonds: Adding Extra Strength
- Triple Bonds: A Symphony of Sigma and Pi
- Visualizing the Triple Bond
- Example: The Nitrogen Molecule (N₂)
- The Significance of Triple Bond Characteristics
- Bond Length and Strength
- Reactivity
- Geometric Considerations
- Beyond Simple Triple Bonds: Exploring Complexity
- Heteroatomic Triple Bonds
- Conjugated Systems
- Aromatic Compounds
- Practical Applications and Real-World Examples
- Organic Chemistry
- Material Science
- Biochemistry
- Conclusion: A Comprehensive Overview
- Latest Posts
- Latest Posts
- Related Post
How Many Sigma and Pi Bonds in a Triple Bond? A Deep Dive into Chemical Bonding
Understanding chemical bonding is fundamental to grasping the behavior of molecules. One key concept within this field involves the different types of bonds, specifically sigma (σ) and pi (π) bonds, which form the backbone of many chemical structures. This article will delve into the specifics of triple bonds, exploring exactly how many sigma and pi bonds are present and why this arrangement is significant. We will examine the intricacies of bond formation, explore examples, and discuss the implications of triple bond characteristics.
The Fundamentals: Sigma and Pi Bonds
Before diving into the complexities of triple bonds, let's refresh our understanding of sigma and pi bonds. These are types of covalent bonds, which are formed by the sharing of electron pairs between atoms. The distinction lies in how the electron orbitals overlap:
Sigma (σ) Bonds: The Foundation
A sigma bond is formed by the direct, head-on overlap of atomic orbitals. This overlap results in a region of high electron density directly between the two bonded nuclei. Sigma bonds are the strongest type of covalent bond and are always the first bond formed when atoms combine. They are found in single, double, and triple bonds.
Pi (π) Bonds: Adding Extra Strength
A pi bond is formed by the sideways overlap of p orbitals. Unlike sigma bonds, the region of high electron density in a pi bond is located above and below the internuclear axis. Pi bonds are weaker than sigma bonds and only form after a sigma bond is already established. They are found in double and triple bonds.
Triple Bonds: A Symphony of Sigma and Pi
Now, let's focus on the topic at hand: triple bonds. A triple bond is a strong covalent bond formed by the sharing of three pairs of electrons between two atoms. Crucially, this arrangement comprises one sigma (σ) bond and two pi (π) bonds.
Key Takeaway: A triple bond always consists of one sigma bond and two pi bonds. This is a fundamental concept in chemistry.
Visualizing the Triple Bond
Imagine two carbon atoms forming a triple bond. One carbon atom's sp hybridized orbital overlaps head-on with the sp hybridized orbital of the other carbon atom, forming a strong sigma bond. Then, the remaining two unhybridized p orbitals on each carbon atom overlap sideways, forming two weaker pi bonds. This creates a strong, relatively short bond length.
Example: The Nitrogen Molecule (N₂)
Nitrogen gas (N₂) is a perfect example to illustrate the concept of a triple bond. Each nitrogen atom has five valence electrons. They share three electron pairs to achieve a stable octet, forming a triple bond (N≡N). This bond comprises one sigma bond and two pi bonds. The triple bond is exceptionally strong, explaining the inertness of nitrogen gas under standard conditions.
The Significance of Triple Bond Characteristics
The presence of a triple bond dramatically impacts the properties of a molecule. This is due to the combination of a strong sigma bond and two weaker pi bonds.
Bond Length and Strength
Triple bonds are characterized by their short bond length and high bond strength. The presence of three bonding electron pairs creates a strong attraction between the atoms, pulling them closer together and making the bond more resistant to breakage. This is reflected in the higher energy required to break a triple bond compared to a single or double bond.
Reactivity
Molecules with triple bonds often exhibit distinct reactivity patterns. The pi bonds, being relatively weaker than the sigma bond, are more susceptible to attack by reagents. This makes molecules containing triple bonds highly reactive in certain reactions, such as addition reactions.
Geometric Considerations
Triple bonds result in linear molecular geometry. This stems from the sp hybridization of the atoms involved in the triple bond. This linear arrangement influences the overall shape and properties of the molecule.
Beyond Simple Triple Bonds: Exploring Complexity
While the basic concept of one sigma and two pi bonds in a triple bond applies to many scenarios, let's explore some nuances:
Heteroatomic Triple Bonds
Triple bonds are not limited to homonuclear diatomic molecules like nitrogen. They also occur in molecules containing different atoms, such as carbon monoxide (CO), where a carbon atom and an oxygen atom are connected by a triple bond. The same fundamental principle applies: one sigma bond and two pi bonds.
Conjugated Systems
In more complex molecules, the presence of alternating single and multiple bonds can lead to delocalized pi electron systems called conjugated systems. While not technically a "triple bond" in the strictest sense, conjugated systems share similar characteristics, exhibiting increased stability and reactivity patterns due to electron delocalization.
Aromatic Compounds
Aromatic compounds, like benzene, possess a ring structure with delocalized pi electrons, contributing to their exceptional stability. While benzene doesn't have triple bonds, the principles of pi bond formation and electron delocalization are fundamental to understanding its properties.
Practical Applications and Real-World Examples
Understanding triple bonds is critical across various fields:
Organic Chemistry
Triple bonds are prevalent in organic chemistry, appearing in alkynes, nitriles, and other functional groups. Their unique reactivity is exploited in a wide array of organic synthesis reactions.
Material Science
Triple bonds play a significant role in the design and synthesis of new materials. The strength and rigidity associated with triple bonds make them valuable in creating polymers, plastics, and other materials with enhanced properties.
Biochemistry
Triple bonds are found in some biomolecules, playing a part in their structure and function. Understanding their properties is important in fields like pharmacology and drug discovery.
Conclusion: A Comprehensive Overview
In conclusion, a triple bond is a crucial aspect of chemical bonding, comprising one strong sigma bond and two weaker pi bonds. This arrangement gives molecules containing triple bonds their distinct characteristics, including short bond lengths, high bond strength, unique reactivity, and linear molecular geometry. Understanding the formation and properties of triple bonds is essential in numerous scientific disciplines, from organic chemistry and material science to biochemistry and beyond. This fundamental knowledge serves as a cornerstone in unraveling the complexities of molecular structure and reactivity. Further exploration into related concepts like hybridization, molecular orbital theory, and reaction mechanisms can provide an even deeper understanding of this fascinating aspect of chemistry.
Latest Posts
Latest Posts
-
The Main Purpose Of Cellular Respiration Is To
Mar 18, 2025
-
Red And White Blood Cells In Fluid Matrix
Mar 18, 2025
-
Why Blood Is Considered A Connective Tissue
Mar 18, 2025
-
How Much Is 1 4 Of A Gallon
Mar 18, 2025
-
Integrated Rate Equation For Zero Order
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Sigma And Pi Bonds In A Triple Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
