Integrated Rate Equation For Zero Order
News Leon
Mar 18, 2025 · 7 min read
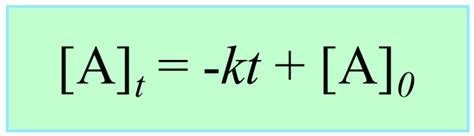
Table of Contents
Integrated Rate Equation for Zero-Order Reactions: A Comprehensive Guide
Understanding reaction kinetics is crucial in various fields, from chemistry and chemical engineering to environmental science and biochemistry. One fundamental aspect of reaction kinetics is determining the rate law, which describes how the rate of a reaction depends on the concentration of reactants. This article delves into the integrated rate equation for zero-order reactions, explaining its derivation, applications, and practical implications. We will explore how to determine the rate constant, predict reactant concentrations over time, and understand the characteristics that define zero-order reactions.
What are Zero-Order Reactions?
A zero-order reaction is a chemical reaction where the rate of reaction is independent of the concentration of the reactants. This seemingly counterintuitive behavior arises from specific reaction mechanisms. The rate of the reaction remains constant throughout the reaction's progress, meaning it doesn't speed up or slow down as the reactants are consumed.
Key Characteristics of Zero-Order Reactions:
- Rate independent of reactant concentration: This is the defining characteristic. Doubling, tripling, or changing the concentration of the reactant(s) has no effect on the reaction rate.
- Constant rate: The reaction proceeds at a constant rate until one of the reactants is completely consumed.
- Linear concentration-time profile: A graph of concentration versus time yields a straight line with a negative slope.
- Often involve surface reactions or catalytic processes: Many zero-order reactions are observed in heterogeneous catalysis where the reaction rate is limited by the availability of active sites on a catalyst's surface, rather than the concentration of the reactants themselves.
- Can exhibit saturation kinetics: At very high reactant concentrations, even reactions that appear zero-order might exhibit a dependence on concentration. This is often because the catalyst sites become saturated.
Deriving the Integrated Rate Law for Zero-Order Reactions
The rate law for a zero-order reaction can be expressed as:
Rate = k
where:
- Rate represents the rate of the reaction (e.g., in units of M/s or mol L⁻¹ s⁻¹).
- k is the rate constant (in units of M/s or mol L⁻¹ s⁻¹), which is specific to the reaction at a given temperature. Note the unusual units for the rate constant, reflecting its role as a constant rate regardless of concentration.
We can also express the rate in terms of the change in reactant concentration over time:
Rate = -d[A]/dt
where:
- [A] represents the concentration of reactant A.
- d[A]/dt represents the change in the concentration of A with respect to time. The negative sign indicates that the concentration of the reactant decreases as the reaction progresses.
Equating these two expressions for the rate, we have:
-d[A]/dt = k
Now, we can separate the variables and integrate to obtain the integrated rate law:
∫d[A] = -k∫dt
Integrating both sides, we get:
[A] = -kt + [A]₀
where:
- [A] is the concentration of reactant A at time t.
- [A]₀ is the initial concentration of reactant A at time t = 0.
This is the integrated rate law for a zero-order reaction. It's a linear equation, revealing the linear relationship between concentration and time.
Applications of the Integrated Rate Equation
The integrated rate law for zero-order reactions has several important applications:
- Determining the rate constant (k): By plotting [A] versus t, a straight line is obtained with a slope of -k and a y-intercept of [A]₀. The negative slope directly yields the rate constant.
- Predicting reactant concentrations: Knowing the initial concentration [A]₀, the rate constant k, and the time t, we can calculate the concentration of reactant A at any time during the reaction using the equation [A] = -kt + [A]₀.
- Determining reaction half-life: The half-life (t₁/₂) is the time it takes for the concentration of a reactant to decrease to half its initial value. For a zero-order reaction, the half-life is given by:
t₁/₂ = [A]₀ / 2k
Notice that the half-life of a zero-order reaction depends on the initial concentration of the reactant. This contrasts with first and second-order reactions where the half-life is independent of the initial concentration.
- Analyzing enzyme-catalyzed reactions: At high substrate concentrations, some enzyme-catalyzed reactions exhibit zero-order kinetics. This occurs when the enzyme is saturated with substrate, meaning that all enzyme active sites are occupied. The reaction rate becomes independent of the substrate concentration, exhibiting zero-order behavior.
Examples of Zero-Order Reactions
While less common than first or second-order reactions, several real-world examples demonstrate zero-order kinetics:
- Enzyme-catalyzed reactions (at high substrate concentrations): As previously mentioned, when the enzyme is saturated with substrate, the reaction rate is independent of substrate concentration.
- Photochemical reactions: The rate of many photochemical reactions depends on the intensity of the light source rather than the concentration of the reactants. If the light intensity is constant, the reaction proceeds at a constant rate, exhibiting zero-order kinetics.
- Gas-phase decomposition on a metal surface: The decomposition of certain gases on a metal surface can follow zero-order kinetics if the surface is saturated with adsorbed reactant molecules. The rate is determined by the number of active sites available, not the concentration of the gas in the bulk phase.
- Certain heterogeneous catalytic reactions: When the reactant is adsorbed onto a catalyst surface before reacting, if the catalyst's surface is completely covered, the reaction rate is independent of the reactant concentration in the bulk phase.
Distinguishing Zero-Order Reactions from Other Reaction Orders
It's crucial to distinguish zero-order reactions from other reaction orders. This is done by analyzing experimental data. Here's a comparison:
| Reaction Order | Rate Law | Integrated Rate Law | Plot | Slope | Intercept | Half-life |
|---|---|---|---|---|---|---|
| Zero-order | Rate = k | [A] = -kt + [A]₀ | [A] vs. t | -k | [A]₀ | [A]₀ / 2k |
| First-order | Rate = k[A] | ln[A] = -kt + ln[A]₀ | ln[A] vs. t | -k | ln[A]₀ | 0.693 / k |
| Second-order | Rate = k[A]² | 1/[A] = kt + 1/[A]₀ | 1/[A] vs. t | k | 1/[A]₀ | 1 / k[A]₀ |
By plotting the appropriate data (e.g., [A] vs. t for zero-order, ln[A] vs. t for first-order, 1/[A] vs. t for second-order), you can determine the reaction order by observing which plot yields a straight line. The slope of the straight line will give you the rate constant.
Advanced Considerations and Limitations
While the integrated rate law for zero-order reactions provides a useful framework, several nuances need consideration:
- Temperature dependence: Like all rate constants, the rate constant (k) for a zero-order reaction is temperature-dependent and follows the Arrhenius equation. Increasing temperature generally increases the rate constant.
- Reaction mechanisms: The underlying reaction mechanism plays a crucial role in determining whether a reaction exhibits zero-order kinetics. Often, zero-order behavior arises from surface reactions or saturation kinetics.
- Validity of the model: The zero-order model is only valid within a certain concentration range. At low concentrations, the reaction might transition to a different order as the surface saturation assumption is no longer valid.
- Complex reactions: Real-world reactions are often complex, involving multiple steps. While a specific step might be zero-order, the overall reaction order might be different.
Conclusion
The integrated rate equation for zero-order reactions, [A] = -kt + [A]₀, provides a powerful tool to understand and analyze reactions that are independent of reactant concentration. Understanding this equation allows for the determination of rate constants, prediction of concentration changes over time, and provides insight into specific reaction mechanisms, especially those involving catalysis or surface reactions. By carefully analyzing experimental data and understanding the limitations of the model, we can effectively utilize the integrated rate law for zero-order reactions to enhance our understanding of reaction kinetics across various scientific disciplines. Remember to always consider the specific reaction conditions and the validity of the zero-order approximation within a defined concentration range.
Latest Posts
Related Post
Thank you for visiting our website which covers about Integrated Rate Equation For Zero Order . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.