How Many Sig Figs In 0.001
News Leon
Mar 24, 2025 · 5 min read
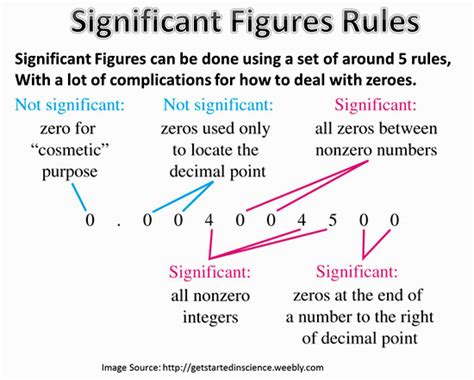
Table of Contents
How Many Significant Figures in 0.001? A Deep Dive into Significant Figures
The question of how many significant figures (sig figs) are in 0.001 is deceptively simple. While the answer might seem immediately obvious to some, a thorough understanding requires a grasp of the underlying principles governing significant figures in scientific notation and measurement. This article will explore this seemingly simple question in detail, providing a comprehensive guide to significant figures and their importance in scientific accuracy and reporting.
Understanding Significant Figures
Significant figures are the digits in a number that carry meaning contributing to its precision. They represent the level of certainty in a measurement. In essence, they tell us how much we know about a value and how much is simply estimated or implied. This is crucial in science and engineering where accuracy and precision are paramount. Incorrect handling of significant figures can lead to significant errors in calculations and conclusions.
The rules for determining significant figures are as follows:
-
All non-zero digits are significant. The number 25 has two significant figures. The number 12345 has five significant figures.
-
Zeros between non-zero digits are significant. The number 101 has three significant figures. The number 2005 has four significant figures.
-
Leading zeros (zeros to the left of the first non-zero digit) are not significant. These zeros only serve to place the decimal point. The number 0.002 has only one significant figure (the 2).
-
Trailing zeros (zeros to the right of the last non-zero digit) are significant only if the number contains a decimal point. The number 100 has one significant figure. However, the number 100. has three significant figures, and the number 100.0 has four.
-
Trailing zeros in a number without a decimal point are ambiguous. To avoid ambiguity, it's best to use scientific notation.
Applying the Rules to 0.001
Now, let's apply these rules to the number 0.001. Notice that the number contains only one non-zero digit (1) and two leading zeros. According to the rules above, leading zeros are not significant. Therefore, 0.001 has only one significant figure.
Scientific Notation and Significant Figures
Scientific notation provides a clear and unambiguous way to represent numbers with the correct number of significant figures. Scientific notation expresses a number as a product of a coefficient (a number between 1 and 10) and a power of 10. For example, 0.001 can be written in scientific notation as 1 x 10⁻³. In this form, the significant figure is immediately apparent – it's the '1' in the coefficient.
Using scientific notation eliminates ambiguity regarding trailing zeros. For example, the number 100 could be written as 1 x 10², indicating one significant figure, 1.0 x 10², indicating two significant figures, or 1.00 x 10², indicating three significant figures. This clear representation prevents misinterpretations that could occur with standard decimal notation.
The Importance of Significant Figures in Calculations
The significance of significant figures extends beyond simply reporting measurements. It's crucial in mathematical operations to ensure the results are not presented with a false sense of precision. When performing calculations involving numbers with different numbers of significant figures, specific rules apply:
-
Addition and Subtraction: The result should have the same number of decimal places as the measurement with the fewest decimal places.
-
Multiplication and Division: The result should have the same number of significant figures as the measurement with the fewest significant figures.
For example, if we add 10.01 (four significant figures) and 1.2 (two significant figures), the result should be rounded to one decimal place (11.2). If we multiply 10.01 (four significant figures) and 1.2 (two significant figures), the result should be rounded to two significant figures (12).
Failing to adhere to these rules during calculations leads to a spurious level of accuracy, implying a precision that is not actually present in the measurements. This can be a significant source of error in scientific studies and engineering projects.
Rounding and Significant Figures
Rounding is an essential aspect of working with significant figures. When a calculation yields more digits than are significant, rounding is necessary to maintain accuracy. The most common method involves looking at the digit immediately to the right of the last significant figure. If this digit is 5 or greater, round up; if it's less than 5, round down. However, more complex rounding rules exist to address certain ambiguities.
For example, if we have the number 12.345 and need to round it to three significant figures, we would round it up to 12.3, since 4 is less than 5. If we need to round 12.355 to three significant figures we would round up to 12.4 (following standard rounding rules).
Real-World Applications of Significant Figures
The concept of significant figures is not merely an academic exercise; it has real-world implications across numerous fields:
-
Engineering: In structural engineering, accurate calculations involving significant figures are crucial for ensuring the stability and safety of buildings and bridges. A minor error in calculations can have catastrophic consequences.
-
Medicine: Precise dosages in medicine depend heavily on the accurate application of significant figures. Incorrect calculation can lead to underdosing or overdosing, both of which can be dangerous.
-
Environmental Science: Analyzing environmental data involves numerous measurements and calculations. Accurate handling of significant figures ensures the reliability of environmental models and predictions.
-
Chemistry and Physics: In laboratory settings, precise measurements are fundamental to experimental design and data interpretation. Understanding significant figures is essential for ensuring the reliability of experimental results.
Avoiding Common Mistakes with Significant Figures
Several common mistakes can occur when dealing with significant figures. Being aware of these pitfalls can improve accuracy:
-
Ignoring leading zeros: Remember that leading zeros are not significant.
-
Misinterpreting trailing zeros: Trailing zeros are only significant if a decimal point is present.
-
Incorrect rounding: Always round appropriately based on the number of significant figures required.
-
Inconsistent application of rules: Make sure to apply the rules for addition/subtraction and multiplication/division consistently.
Conclusion
The number 0.001 possesses only one significant figure. Understanding significant figures is critical for anyone working with numerical data, particularly in scientific and engineering fields. Proper application of these rules is essential for accurate measurements, reliable calculations, and credible scientific communication. By carefully considering the principles of significant figures and consistently applying the correct rules, we can ensure greater precision and reduce the risk of errors in our work. The seemingly simple question of significant figures in 0.001 unveils a deeper understanding of precision, accuracy, and the importance of clear communication in scientific reporting.
Latest Posts
Latest Posts
-
The Most Powerful Countries In 1900
Mar 29, 2025
-
Which Of The Following Gives Rise To Epithelial Tissues Only
Mar 29, 2025
-
Arrange The Following Carboxylic Acids In Order Of Acidity
Mar 29, 2025
-
What Is The Mass Of 1 Molecule Of Water
Mar 29, 2025
-
What Does Isalpha Do In Python
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Sig Figs In 0.001 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
