How Many Protons And Electrons In Magnesium
News Leon
Mar 26, 2025 · 5 min read
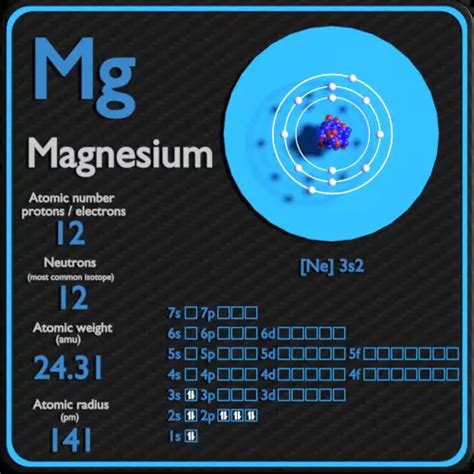
Table of Contents
How Many Protons and Electrons in Magnesium? A Deep Dive into Atomic Structure
Magnesium, a vital element for human health and a cornerstone of numerous industrial applications, holds a fascinating place in the periodic table. Understanding its atomic structure, particularly the number of protons and electrons, is key to grasping its chemical properties and behaviour. This comprehensive guide delves into the intricacies of magnesium's atomic composition, exploring its electron configuration, isotopic variations, and the broader implications of its proton-electron balance.
Understanding Atomic Structure: Protons, Electrons, and Neutrons
Before focusing specifically on magnesium, let's establish a foundational understanding of atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines an element's atomic number and uniquely identifies it on the periodic table.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. Electrons are significantly lighter than protons and are involved in chemical bonding. In a neutral atom, the number of electrons equals the number of protons.
-
Neutrons: Neutrally charged particles also located in the nucleus. They contribute to the atom's mass but don't directly participate in chemical reactions. The number of neutrons can vary within an element, leading to isotopes.
Magnesium's Atomic Number and Electron Configuration
Magnesium (Mg), located in Group 2 (alkaline earth metals) and Period 3 of the periodic table, has an atomic number of 12. This crucial number tells us that a neutral magnesium atom contains 12 protons. Because the atom is neutral, it also possesses 12 electrons.
The arrangement of these 12 electrons in electron shells determines magnesium's chemical reactivity. Following the principles of electron configuration, these electrons are distributed as follows:
- First shell (n=1): 2 electrons
- Second shell (n=2): 8 electrons
- Third shell (n=3): 2 electrons
This configuration is often represented as 1s²2s²2p⁶3s². The outermost shell, containing two electrons, is known as the valence shell. These valence electrons are responsible for magnesium's chemical bonding behaviour. Magnesium readily loses these two valence electrons to achieve a stable, noble gas electron configuration similar to neon (1s²2s²2p⁶). This tendency makes magnesium highly reactive, particularly with nonmetals and oxidizing agents.
Magnesium's Chemical Reactivity and Bonding
The readiness of magnesium to lose its two valence electrons explains its characteristic chemical properties:
-
Formation of ionic compounds: Magnesium readily forms ionic bonds with electronegative elements like oxygen (forming MgO) and chlorine (forming MgCl₂). In these compounds, magnesium exists as a +2 cation (Mg²⁺).
-
Reducing agent: Because of its tendency to lose electrons, magnesium acts as a strong reducing agent in many chemical reactions.
-
Metallic bonding: Magnesium's metallic nature stems from the delocalization of its valence electrons, forming a "sea" of electrons that holds the positively charged magnesium ions together in a metallic lattice. This explains magnesium's properties like electrical conductivity and malleability.
Isotopes of Magnesium: Variations in Neutron Number
While the number of protons and electrons remains consistent in a neutral magnesium atom, the number of neutrons can vary. This leads to the existence of different isotopes of magnesium. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. The most common isotopes of magnesium are:
-
Magnesium-24 (²⁴Mg): This is the most abundant isotope, comprising approximately 79% of naturally occurring magnesium. It contains 12 protons, 12 electrons, and 12 neutrons.
-
Magnesium-25 (²⁵Mg): This isotope makes up about 10% of natural magnesium. It has 12 protons, 12 electrons, and 13 neutrons.
-
Magnesium-26 (²⁶Mg): This is the least abundant natural isotope, making up roughly 11% of naturally occurring magnesium. It contains 12 protons, 12 electrons, and 14 neutrons.
Although isotopes have different numbers of neutrons, their chemical properties remain largely unchanged because the number of protons and electrons, which dictate chemical behavior, are identical. However, their physical properties, such as mass, can vary.
Applications of Magnesium: From Biology to Industry
Understanding the atomic structure of magnesium is crucial for appreciating its diverse applications:
-
Biological Roles: Magnesium is an essential mineral for human health, playing a vital role in numerous enzymatic reactions, muscle contraction, nerve function, and maintaining healthy bones.
-
Alloying Agent: Magnesium's lightweight nature and relatively high strength make it an ideal alloying agent in the aerospace, automotive, and electronics industries. Magnesium alloys are used in aircraft parts, car components, and laptop casings.
-
Reducing Agent: Its reducing properties are utilized in various chemical processes, including the production of titanium and other metals.
-
Fire Retardants: Magnesium compounds are used as fire retardants due to their ability to absorb heat and release inert gases.
Conclusion: The Significance of Proton-Electron Balance in Magnesium
The precise number of protons and electrons in magnesium—12 each in a neutral atom—is fundamental to its chemical and physical properties. The arrangement of electrons in shells dictates its reactivity, leading to the formation of ionic compounds, its function as a reducing agent, and its metallic bonding. While the number of neutrons can vary, resulting in different isotopes, these variations don't fundamentally alter the element's chemical behavior. The understanding of magnesium's atomic structure underpins its diverse applications across various fields, highlighting the importance of this element in both biological and industrial contexts. Further research continues to unveil new aspects of magnesium's behavior and potential applications, demonstrating the ongoing significance of studying its atomic structure. The interplay between protons and electrons remains a cornerstone of chemistry and a key to unlocking further scientific advancements.
Latest Posts
Latest Posts
-
Which Of The Following Combinations Are Correctly Matched
Mar 28, 2025
-
The Figure Gives An Overhead View Of The Path
Mar 28, 2025
-
A Galvanometer Has A Resistance Of 20 Ohm
Mar 28, 2025
-
What Are The Coordinates Of Point Q
Mar 28, 2025
-
30 As A Product Of Prime Factors
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons And Electrons In Magnesium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
