How Many Oxygen Molecules Can One Hemoglobin Carry
News Leon
Mar 18, 2025 · 5 min read
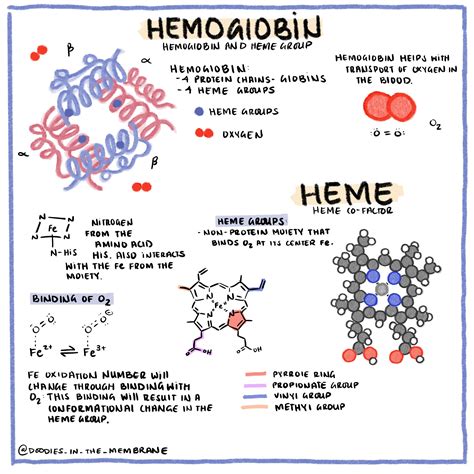
Table of Contents
How Many Oxygen Molecules Can One Hemoglobin Carry?
Understanding the oxygen-carrying capacity of hemoglobin is crucial to comprehending respiratory physiology and various related health conditions. This article delves deep into the intricate relationship between hemoglobin and oxygen, exploring the molecular mechanics behind oxygen transport and the factors influencing its efficiency. We will uncover precisely how many oxygen molecules a single hemoglobin molecule can carry, and delve into the implications of this capacity for human health.
The Hemoglobin Molecule: A Marvel of Biological Engineering
Hemoglobin, a metalloprotein found abundantly in red blood cells (erythrocytes), is the primary oxygen transporter in the circulatory system. Its tetrameric structure – comprised of four globin subunits – is the key to its remarkable oxygen-binding capabilities. Each subunit cradles a heme group, a porphyrin ring complex containing a ferrous ion (Fe²⁺). This iron atom is the critical component that binds oxygen.
The Structure-Function Relationship
The precise arrangement of these subunits and heme groups is not arbitrary. The quaternary structure of hemoglobin, a consequence of interactions between its constituent subunits (typically two alpha and two beta subunits in adult hemoglobin, HbA), exhibits allosteric properties. This means that the binding of oxygen to one heme group influences the oxygen affinity of the remaining heme groups. This cooperative binding is vital for efficient oxygen uptake in the lungs and release in the tissues.
The Heme Group: The Oxygen Binding Site
The heme group, nestled within each globin subunit, acts as the oxygen binding site. The ferrous iron (Fe²⁺) at the center of the heme group readily forms a reversible bond with an oxygen molecule (O₂). This bond formation doesn't involve a permanent change in the iron's oxidation state; it remains ferrous. The ability of iron to reversibly bind oxygen is paramount for its function as an oxygen transporter. The process is highly dynamic, allowing hemoglobin to pick up oxygen in oxygen-rich environments (like the lungs) and release it in oxygen-poor environments (like the tissues).
The Magic Number: Four Oxygen Molecules
The answer to the central question – how many oxygen molecules can one hemoglobin molecule carry? – is four. Because each of the four heme groups within a single hemoglobin molecule can bind one oxygen molecule, the maximum oxygen-carrying capacity of a hemoglobin molecule is four O₂ molecules.
Cooperative Binding: Enhancing Efficiency
The cooperative binding of oxygen to hemoglobin is a crucial feature that enhances its efficiency as an oxygen transporter. The binding of the first oxygen molecule to a heme group induces a conformational change in the hemoglobin molecule, increasing the affinity of the remaining heme groups for oxygen. This means that subsequent oxygen molecules bind more readily. Conversely, the release of oxygen from one heme group facilitates the release of oxygen from the other heme groups. This positive cooperativity ensures efficient oxygen uptake in the lungs where partial pressure of oxygen is high and efficient oxygen release in the tissues where partial pressure of oxygen is low.
The Oxygen-Hemoglobin Dissociation Curve
The relationship between the partial pressure of oxygen (pO₂) and the percentage of hemoglobin saturation with oxygen is graphically represented by the oxygen-hemoglobin dissociation curve (also known as the oxyhemoglobin dissociation curve). This sigmoid curve reflects the cooperative nature of oxygen binding. The curve's shape illustrates that at higher pO₂ (like in the lungs), hemoglobin readily binds oxygen, achieving near-saturation. As pO₂ decreases (like in the tissues), hemoglobin releases oxygen, making it available for cellular respiration.
Factors Affecting Hemoglobin's Oxygen-Carrying Capacity
Several factors can influence the oxygen-carrying capacity of hemoglobin, impacting the efficiency of oxygen transport throughout the body. These factors can shift the oxygen-hemoglobin dissociation curve to the right or left, altering the affinity of hemoglobin for oxygen.
pH (Bohr Effect):
A decrease in pH (increased acidity) reduces hemoglobin's affinity for oxygen, shifting the curve to the right. This is known as the Bohr effect. In actively metabolizing tissues, the increased production of carbon dioxide leads to a lower pH, promoting oxygen release from hemoglobin.
Temperature:
Increased temperature also reduces hemoglobin's affinity for oxygen, shifting the curve to the right. Active tissues are warmer, and this increased temperature contributes to oxygen unloading.
2,3-Bisphosphoglycerate (2,3-BPG):
2,3-BPG, a molecule present in red blood cells, binds to hemoglobin and reduces its affinity for oxygen, shifting the curve to the right. Increased levels of 2,3-BPG, often seen in high-altitude adaptation or certain anemias, enhance oxygen release in the tissues.
Carbon Dioxide:
Carbon dioxide itself can directly bind to hemoglobin, reducing its affinity for oxygen and shifting the curve to the right. This is another contributing factor to efficient oxygen unloading in the tissues.
Clinical Implications: Understanding Hemoglobin Function in Disease
Disruptions in hemoglobin function can have significant clinical implications. Understanding the oxygen-carrying capacity of hemoglobin is crucial for diagnosing and managing various hematological disorders.
Anemia:
Anemia, characterized by a deficiency of red blood cells or hemoglobin, directly reduces the blood's oxygen-carrying capacity. Various types of anemia, including iron-deficiency anemia, sickle cell anemia, and thalassemia, can significantly impair oxygen transport, leading to fatigue, shortness of breath, and other symptoms.
Carbon Monoxide Poisoning:
Carbon monoxide (CO) has a much higher affinity for hemoglobin than oxygen. CO binding to hemoglobin forms carboxyhemoglobin, effectively reducing the oxygen-carrying capacity of the blood. This can lead to severe hypoxia (oxygen deficiency) and even death.
Altitude Sickness:
At high altitudes, the partial pressure of oxygen is lower. The body compensates by increasing the production of 2,3-BPG, enhancing oxygen release from hemoglobin in the tissues. However, this may not be sufficient, and altitude sickness can occur.
Conclusion: The Vital Role of Hemoglobin in Oxygen Transport
Hemoglobin, with its remarkable ability to bind and transport four oxygen molecules per molecule, plays a vital role in maintaining oxygen supply to the body's tissues. The cooperative binding of oxygen, influenced by factors like pH, temperature, and 2,3-BPG, ensures efficient oxygen uptake and release, tailored to the metabolic needs of the tissues. Understanding the intricate mechanisms of hemoglobin function is crucial for comprehending respiratory physiology and the pathogenesis of various related diseases. Future research continues to unravel the intricacies of hemoglobin, paving the way for novel therapeutic strategies for treating oxygen transport disorders. The fascinating story of hemoglobin and its oxygen-carrying capacity remains a captivating area of biomedical investigation.
Latest Posts
Latest Posts
-
Oxidation Number Of O In H2o
Mar 19, 2025
-
Largest Cell Of The Human Body
Mar 19, 2025
-
Sin X Cos X 2 1 Sin 2x
Mar 19, 2025
-
Find The Surface Area Of The Square Pyramid Shown Below
Mar 19, 2025
-
A Cell In A Hypertonic Solution Will
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Oxygen Molecules Can One Hemoglobin Carry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
