Oxidation Number Of O In H2o
News Leon
Mar 19, 2025 · 6 min read
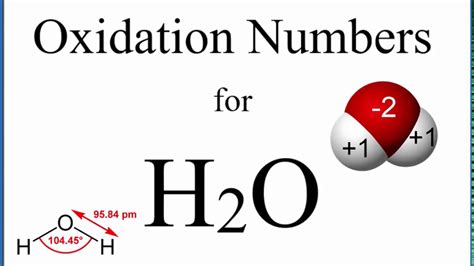
Table of Contents
Determining the Oxidation Number of Oxygen in H₂O: A Comprehensive Guide
The seemingly simple water molecule, H₂O, offers a valuable opportunity to understand the fundamental concept of oxidation numbers. While the answer might seem straightforward, a deeper dive reveals nuances and broader implications for understanding chemical bonding and reactivity. This article will explore the oxidation number of oxygen in H₂O, explaining the rules, exceptions, and applications of this crucial concept in chemistry.
Understanding Oxidation Numbers
Before diving into the specifics of H₂O, let's establish a firm understanding of oxidation numbers themselves. The oxidation number, also known as oxidation state, is a number assigned to an atom in a chemical compound that represents the hypothetical charge that atom would have if all bonds to atoms of different elements were 100% ionic. This means we're assigning charges based on an idealized scenario, not necessarily reflecting the actual charge distribution in the molecule.
It's crucial to remember that oxidation numbers are a bookkeeping tool; they are not real charges. They help us track electron transfer in redox reactions and predict the behavior of elements in various chemical environments.
Rules for Assigning Oxidation Numbers
Several rules guide the assignment of oxidation numbers. These rules, applied sequentially, help determine the oxidation state of each atom in a compound.
Key Rules:
-
The oxidation number of an atom in its elemental form is zero. For example, the oxidation number of O₂ is 0, and the oxidation number of H₂ is 0.
-
The oxidation number of a monatomic ion is equal to its charge. For instance, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
-
The oxidation number of hydrogen is generally +1, except in metal hydrides where it is -1. In most compounds, hydrogen loses one electron to achieve a stable electron configuration. However, in metal hydrides (e.g., NaH, LiH), hydrogen gains an electron to achieve a stable configuration.
-
The oxidation number of oxygen is generally -2, except in peroxides where it is -1 and in superoxides where it is -1/2. Oxygen is highly electronegative and tends to gain two electrons to achieve a stable octet. However, exceptions exist in peroxides (e.g., H₂O₂) and superoxides (e.g., KO₂).
-
The sum of oxidation numbers of all atoms in a neutral molecule is zero.
-
The sum of oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
Determining the Oxidation Number of Oxygen in H₂O
Applying the rules above to H₂O, we can easily determine the oxidation number of oxygen.
-
Hydrogen's oxidation number: Based on rule 3, the oxidation number of hydrogen in H₂O is +1. Since there are two hydrogen atoms, the total contribution from hydrogen is +2.
-
Overall charge: Water (H₂O) is a neutral molecule, so the sum of oxidation numbers must be zero (rule 5).
-
Oxygen's oxidation number: Let 'x' represent the oxidation number of oxygen. Therefore, we can set up the equation: (+1) + (+1) + x = 0. Solving for x, we find that x = -2.
Therefore, the oxidation number of oxygen in H₂O is -2.
Exceptions and Considerations
While the oxidation number of oxygen in H₂O is typically -2, it's essential to be aware of potential exceptions and nuances. These exceptions primarily arise when oxygen forms bonds with elements that are less electronegative than itself.
Peroxides:
In peroxides (like hydrogen peroxide, H₂O₂), the oxygen-oxygen bond features a single bond with each oxygen atom possessing one unpaired electron. Each oxygen atom effectively shares one electron with the other oxygen atom, leading to an oxidation state of -1 for each oxygen atom.
Superoxides:
In superoxides (like potassium superoxide, KO₂), the oxygen atoms form a superoxide anion (O₂⁻), with each oxygen atom exhibiting an oxidation state of -1/2.
Fluorine Compounds:
Oxygen's electronegativity is surpassed only by fluorine. In compounds containing oxygen and fluorine, oxygen can display a positive oxidation state. For example, in oxygen difluoride (OF₂), oxygen's oxidation number is +2.
Applications of Oxidation Numbers
The concept of oxidation numbers has numerous applications across various chemical fields:
-
Redox Reactions: Oxidation numbers are essential in identifying redox reactions, where electron transfer occurs. An increase in oxidation number signifies oxidation (loss of electrons), while a decrease signifies reduction (gain of electrons).
-
Balancing Redox Equations: The half-reaction method, a common technique for balancing redox equations, relies heavily on oxidation numbers.
-
Predicting Chemical Reactivity: Oxidation states can help predict the reactivity of different compounds and elements. Elements with high oxidation numbers are often strong oxidizing agents, while those with low oxidation numbers are often strong reducing agents.
-
Nomenclature: Oxidation numbers play a role in the naming of inorganic compounds, helping to distinguish different oxidation states of the same element.
-
Electrochemistry: Oxidation numbers are crucial in understanding electrochemical processes, such as those occurring in batteries and fuel cells.
Beyond the Basics: Delving Deeper into Chemical Bonding in H₂O
While oxidation numbers provide a useful framework for understanding electron distribution, it’s important to remember that they represent a simplification of the actual bonding in H₂O. The bonds in water are covalent, meaning electrons are shared between atoms, rather than completely transferred as implied by the ionic model used in calculating oxidation states.
The electronegativity difference between oxygen and hydrogen leads to a polar covalent bond. Oxygen, being more electronegative, attracts the shared electrons more strongly, resulting in a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms. This polarity is crucial to water's unique properties, such as its high boiling point, surface tension, and ability to act as a solvent.
Further Exploration and Related Concepts
Understanding oxidation numbers forms a foundation for more advanced chemical concepts. This includes:
-
Formal Charge: Formal charge is another method for assigning charges to atoms within a molecule. Unlike oxidation numbers, formal charges consider the number of valence electrons and the number of bonds and lone pairs associated with each atom.
-
Coordination Chemistry: Oxidation numbers are fundamental in understanding coordination complexes, where metal ions are surrounded by ligands. The oxidation state of the central metal ion significantly influences the properties of the complex.
-
Organometallic Chemistry: In organometallic compounds, which contain both metal and carbon atoms, oxidation states help describe the electron distribution and reactivity of the molecule.
Conclusion
The oxidation number of oxygen in H₂O is definitively -2, a conclusion reached by applying the established rules for assigning oxidation numbers. While a simplified model, this concept is invaluable for understanding chemical reactions, predicting reactivity, and balancing redox equations. It's vital to remember the limitations of this model, particularly regarding the true nature of covalent bonding, but its utility in various chemical applications remains undeniable. A firm grasp of oxidation numbers serves as a strong foundation for further exploration of more complex chemical concepts and reactions.
Latest Posts
Latest Posts
-
In The Figure Pq Is Parallel To Rs
Mar 19, 2025
-
I Am Who I Am Essay
Mar 19, 2025
-
Inertia Is A Property Of Matter
Mar 19, 2025
-
Which Chambers Of The Heart Have Thicker Walls
Mar 19, 2025
-
Which Of The Following Is A Product Of Glycolysis
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of O In H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
