How Many Neutrons Does Uranium 238 Have
News Leon
Mar 24, 2025 · 6 min read
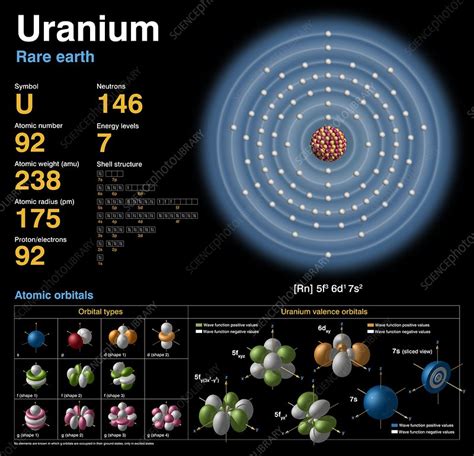
Table of Contents
How Many Neutrons Does Uranium-238 Have? A Deep Dive into Nuclear Physics
Uranium-238, often denoted as ²³⁸U, is a heavy metal isotope that plays a significant role in nuclear physics, geology, and various industrial applications. Understanding its nuclear composition, particularly the number of neutrons, is crucial to comprehending its properties and behavior. This article delves deep into the question: How many neutrons does uranium-238 have? and explores related concepts in nuclear science.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we can determine the number of neutrons in uranium-238, we need to grasp the fundamental building blocks of an atom: protons, neutrons, and electrons.
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element; all uranium atoms have 92 protons.
- Neutrons: Neutrally charged particles residing in the nucleus alongside protons. They contribute to the atom's mass but not its charge. The number of neutrons can vary within the same element, leading to different isotopes.
- Electrons: Negatively charged particles orbiting the nucleus. They are much lighter than protons and neutrons and determine the atom's chemical properties. In a neutral atom, the number of electrons equals the number of protons.
Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This difference in neutron number alters the atom's mass and can significantly impact its stability and radioactivity. Uranium-238 is just one isotope of uranium; others include uranium-235 and uranium-234.
Calculating the Number of Neutrons in Uranium-238
The number following the element's name in an isotope's designation (e.g., 238 in uranium-238) represents the mass number (A). The mass number is the sum of protons (Z) and neutrons (N) in the nucleus:
A = Z + N
Since uranium's atomic number (number of protons) is 92, we can calculate the number of neutrons in uranium-238 as follows:
- A (mass number) = 238
- Z (atomic number) = 92
- N (number of neutrons) = A - Z = 238 - 92 = 146
Therefore, uranium-238 has 146 neutrons.
The Significance of Neutron Number in Uranium-238
The large number of neutrons in uranium-238 is directly related to its properties:
- Radioactivity: Uranium-238 is a radioactive isotope, meaning it undergoes radioactive decay over time. This decay is primarily through alpha decay, where it emits an alpha particle (two protons and two neutrons). The large neutron-to-proton ratio contributes to its instability.
- Nuclear Fission: While uranium-238 itself is not easily fissile (doesn't readily undergo nuclear fission), it can capture neutrons and transform into plutonium-239, which is fissile. This process is crucial in breeder reactors, where more fissile material is produced than consumed.
- Nuclear Applications: Uranium-238's properties make it useful in various applications, including:
- Depleted Uranium: Uranium with a reduced concentration of uranium-235 is called depleted uranium. It's dense and used in armor-piercing munitions.
- Nuclear Fuel: Though less fissile than uranium-235, uranium-238 contributes to the overall energy production in nuclear reactors.
- Radioactive Dating: Uranium-238's decay pattern allows scientists to determine the age of rocks and geological formations through uranium-lead dating.
Comparison with Other Uranium Isotopes
Let's compare the neutron counts of other common uranium isotopes:
- Uranium-235 (²³⁵U): This isotope has 92 protons and 143 neutrons (235 - 92 = 143). It is fissile and plays a critical role in nuclear power generation. The lower neutron-to-proton ratio makes it more susceptible to fission compared to uranium-238.
- Uranium-234 (²³⁴U): This isotope has 92 protons and 142 neutrons (234 - 92 = 142). It is also radioactive and decays through alpha decay.
The difference in neutron numbers among these isotopes leads to variations in their nuclear properties and applications.
The Role of the Strong Nuclear Force
The stability of an atomic nucleus, particularly one with a high number of protons and neutrons like uranium-238, depends on the balance between the electromagnetic repulsive force (between protons) and the strong nuclear force. The strong nuclear force is a short-range attractive force that holds protons and neutrons together within the nucleus. In uranium-238, the strong nuclear force successfully counteracts the electrostatic repulsion between the numerous protons, but only partially. This explains why it's radioactive.
Nuclear Stability and the Neutron-to-Proton Ratio
The neutron-to-proton ratio (N/Z) is a crucial factor in determining the stability of an atomic nucleus. For lighter elements, a N/Z ratio close to 1 is generally indicative of stability. However, for heavier elements like uranium, a higher N/Z ratio is required to maintain stability. The excess neutrons help to overcome the repulsive forces between protons. The large neutron number in uranium-238 is a direct consequence of this need for stability in a nucleus with a high number of protons.
Advanced Concepts: Nuclear Shell Model and Magic Numbers
The nuclear shell model provides a more sophisticated understanding of nuclear structure and stability. This model suggests that neutrons and protons occupy distinct energy levels or shells within the nucleus. Certain numbers of neutrons or protons (called "magic numbers") correspond to particularly stable nuclear configurations. While uranium-238 doesn't have magic numbers of both protons and neutrons, its relatively high number of neutrons contributes to a degree of stability, albeit temporary due to its radioactivity.
Applications Beyond Nuclear Power: Uranium-238's Diverse Uses
Uranium-238's unique properties extend beyond its role in nuclear power generation. It has found applications in various fields:
- Radiation Shielding: Its high density makes it effective in shielding against radiation.
- Kinetic Energy Penetrators: Depleted uranium's high density and self-sharpening properties make it suitable for use in armor-piercing ammunition.
- Nuclear Medicine: While not directly used in diagnostics or therapy, its decay products can be utilized in certain medical applications.
- Geological Dating: Uranium-238 decay is fundamental to radiometric dating techniques.
Conclusion: Understanding the Importance of Uranium-238's Neutron Count
The answer to "How many neutrons does uranium-238 have?" is 146. This seemingly simple number has profound implications in nuclear physics and beyond. Understanding the number of neutrons in uranium-238 is crucial for grasping its radioactivity, its role in nuclear fission, its diverse applications, and the fundamental principles governing the structure and stability of atomic nuclei. The large neutron-to-proton ratio in uranium-238 is directly related to its instability and its unique properties, which have shaped its importance in various scientific and technological fields. This article provides a comprehensive overview, but further exploration into the intricacies of nuclear physics can reveal even more about this fascinating isotope.
Latest Posts
Latest Posts
-
What Is The Molecular Weight Of Oxygen
Mar 26, 2025
-
How Many Nano Second In One Second
Mar 26, 2025
-
Which Of The Following Heterocycles Is Not Aromatic
Mar 26, 2025
-
An Amino Acid At Its Isoelectric Point Is
Mar 26, 2025
-
What Is Difference Between Cilia And Flagella
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Uranium 238 Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
