An Amino Acid At Its Isoelectric Point Is
News Leon
Mar 26, 2025 · 6 min read
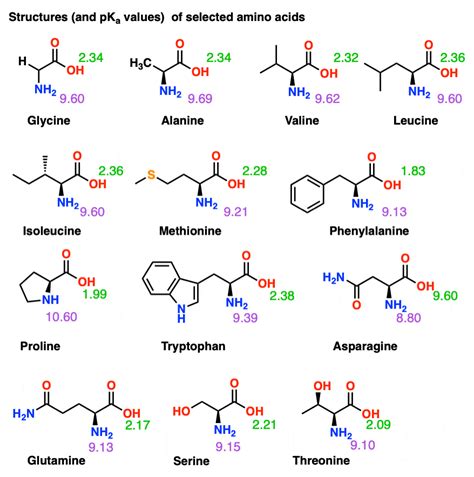
Table of Contents
An Amino Acid at Its Isoelectric Point: A Deep Dive into Zwitterions and Their Significance
Amino acids, the fundamental building blocks of proteins, possess unique chemical properties that dictate their behavior in various biological contexts. A crucial aspect of their characterization lies in understanding their isoelectric point (pI), the pH at which the net charge of an amino acid is zero. This article delves into the intricacies of an amino acid at its isoelectric point, exploring the concept of zwitterions, their significance in protein structure and function, and the practical applications of this knowledge.
Understanding the Isoelectric Point (pI)
The isoelectric point (pI) represents the pH at which a molecule carries no net electrical charge. For amino acids, this is achieved when the positive and negative charges on the molecule precisely balance each other. Amino acids possess at least two ionizable groups: the carboxyl group (-COOH) and the amino group (-NH2). Some amino acids, like aspartic acid and lysine, also have ionizable side chains, adding complexity to their pI calculation.
At a pH below the pI, the amino acid exists predominantly in its cationic form, carrying a net positive charge. Conversely, at a pH above the pI, the amino acid predominantly exists in its anionic form, carrying a net negative charge. At the pI itself, the molecule exists as a zwitterion.
The Zwitterion: A Neutral but Dipolar Species
A zwitterion (from German, meaning "hybrid ion") is a molecule that contains both positive and negative charges, but has a net charge of zero. In the context of amino acids, this means the carboxyl group is deprotonated (-COO⁻) and the amino group is protonated (-NH₃⁺). The zwitterionic form is the predominant species at the pI.
It's crucial to understand that even though the net charge is zero, the zwitterion is not electrically neutral. It possesses distinct regions of positive and negative charge, making it a dipolar molecule. This dipolar nature profoundly impacts the amino acid's physical and chemical properties, including its solubility, electrophoretic mobility, and interaction with other molecules.
Calculating the pI: A Step-by-Step Guide
Calculating the pI requires knowledge of the pKa values of the ionizable groups. The pKa is the pH at which half of the molecules of a given species are deprotonated. For amino acids with only ionizable carboxyl and amino groups, the pI is simply the average of the pKa values of the carboxyl group and the amino group:
pI = (pKa₁ + pKa₂)/2
Where pKa₁ is the pKa of the carboxyl group and pKa₂ is the pKa of the amino group.
However, for amino acids with ionizable side chains, the calculation becomes more complex. The pI is then determined by averaging the pKa values of the two groups that are closest in pH when the molecule is in its zwitterionic form. This often involves considering the pKa of the side chain. Different approaches may be used depending on the specific amino acid.
The Significance of the Isoelectric Point
The isoelectric point is not merely an abstract concept; it holds immense significance in various biological and practical applications:
1. Protein Purification and Separation: Isoelectric Focusing
Isoelectric focusing (IEF) is a powerful technique used to separate proteins based on their pI. A pH gradient is established, and proteins migrate until they reach their respective pI, where their net charge is zero and they stop migrating. This technique is crucial in proteomics and other biochemical research for separating complex mixtures of proteins.
2. Protein Solubility and Stability: The Impact of pH
The solubility of an amino acid or protein is significantly influenced by the pH of the solution. At the pI, the net charge is zero, reducing electrostatic repulsion between molecules, leading to reduced solubility and potentially even precipitation. This principle is exploited in protein purification processes to precipitate unwanted proteins. Understanding the pI is crucial for optimizing protein stability and preventing aggregation.
3. Protein-Protein Interactions: Charge Complementarity
The charge of amino acid residues plays a significant role in protein-protein interactions. The complementary charges of interacting residues can stabilize protein complexes. The pI of individual proteins and the pH of their environment affect the overall charge distribution, thus influencing the formation and stability of protein complexes.
4. Enzyme Activity and Regulation: The Role of the Active Site
The pI of an enzyme's active site significantly influences its catalytic activity. The optimal pH for enzyme activity often corresponds to the pI of the active site. Deviations from the optimal pH can alter the charge distribution in the active site, disrupting substrate binding and catalytic efficiency. Many enzymes function within a narrow pH range dictated by their pI.
5. Drug Design and Development: Targeting Specific Amino Acid Residues
The pI of amino acid residues in drug target proteins is a valuable consideration in drug design. Drugs often interact with specific amino acid residues within the target protein. Understanding the pI of these residues helps design drugs that effectively interact and modulate the protein's activity.
Beyond the Basics: Advanced Concepts
While the basic concept of the pI is relatively straightforward, several more nuanced aspects warrant consideration:
Microheterogeneity and Post-Translational Modifications
The pI of a protein is not always a fixed value. Post-translational modifications (PTMs), such as phosphorylation or glycosylation, can alter the charge of amino acid residues, thereby shifting the protein's pI. Furthermore, microheterogeneity in the protein sequence, caused by genetic variations or incomplete processing, can also lead to variations in the pI.
The Influence of Salt Concentration and Other Ions
The presence of salts and other ions in the solution can significantly affect the pI. These ions can interact with the charged groups on the amino acid or protein, altering their apparent pKa values and therefore the pI.
Predicting pI using Bioinformatic Tools
Several bioinformatic tools are available for predicting the pI of proteins based on their amino acid sequences. These tools are valuable for researchers investigating protein properties and designing experiments. However, the predictions should be considered as estimates; the actual pI may vary slightly depending on experimental conditions.
Practical Applications and Future Directions
The understanding of amino acids at their isoelectric point has numerous applications:
- Food science: pI is crucial in food processing, influencing the texture and stability of food products.
- Cosmetics and personal care: The pI of proteins used in cosmetics affects their stability and interaction with skin.
- Biotechnology: pI is used in various biotechnological processes, including protein purification, enzymatic reactions, and biopharmaceutical development.
- Medical diagnostics: pI-based methods are used in clinical settings for protein analysis and disease diagnosis.
Future research will likely focus on:
- Developing more accurate and sophisticated computational methods for predicting pI, especially considering the effects of PTMs and environmental factors.
- Exploring the role of pI in more complex biological systems, including protein-protein interactions, cellular signaling, and disease pathogenesis.
- Developing novel applications of pI-based technologies in various fields, such as personalized medicine and nanobiotechnology.
Conclusion
The isoelectric point is a fundamental property of amino acids and proteins that profoundly influences their behavior and function. Understanding the concept of zwitterions, the significance of pI in various contexts, and the diverse practical applications of this knowledge are crucial for researchers and professionals across numerous scientific disciplines. As our understanding of this critical parameter deepens, so too will the innovative applications of this knowledge in diverse fields, shaping the future of biotechnology, medicine, and other related areas. This comprehensive understanding facilitates advancements in diverse scientific fields, shaping the future of research and technological innovation.
Latest Posts
Latest Posts
-
How Many Unpaired Electrons Does Cobalt Have
Mar 29, 2025
-
Which Of The Following Is Not Output Device
Mar 29, 2025
-
Which Of The Following Bodies Has The Largest Kinetic Energy
Mar 29, 2025
-
A Cell That Contains One Set Of Chromosomes
Mar 29, 2025
-
An Electric Vehicle Starts From Rest And Accelerates
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about An Amino Acid At Its Isoelectric Point Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
