Which Of The Following Heterocycles Is Not Aromatic
News Leon
Mar 26, 2025 · 5 min read
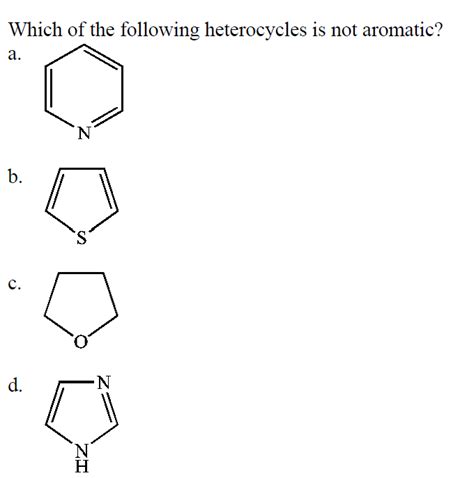
Table of Contents
Which of the Following Heterocycles is Not Aromatic? A Deep Dive into Aromaticity
Aromatic compounds are a fascinating class of organic molecules characterized by their unique stability and reactivity. Understanding aromaticity is crucial for predicting chemical behavior and designing new materials. This article will delve into the criteria for aromaticity and examine several heterocycles, ultimately determining which among them fails to meet the requirements for aromatic character. We will explore the Huckel's rule, the importance of planarity, and the role of conjugation in establishing aromaticity.
Understanding Aromaticity: The Huckel's Rule and Beyond
Before we analyze specific heterocycles, let's establish a firm foundation in the principles of aromaticity. A compound is considered aromatic if it adheres to the following criteria:
- Cyclic: The molecule must be a closed ring structure.
- Planar: All atoms in the ring must lie in the same plane. This allows for effective overlap of p-orbitals.
- Conjugated: The ring must possess a continuous system of overlapping p-orbitals above and below the plane of the ring. This continuous conjugation is essential for delocalization of electrons.
- Huckel's Rule: The molecule must contain (4n + 2) π electrons, where 'n' is a non-negative integer (0, 1, 2, 3...). This rule dictates the number of π electrons required for stability.
Any deviation from these rules typically results in a non-aromatic or anti-aromatic compound. Anti-aromatic compounds are highly unstable due to their 4n π electron count.
Analyzing Common Heterocycles: A Case-by-Case Examination
Let's now examine several common heterocycles and assess their aromaticity based on the criteria outlined above. We will focus on identifying the heterocycle that does not meet the requirements for aromaticity.
1. Pyrrole: An Aromatic Heterocycle
Pyrrole is a five-membered heterocycle containing one nitrogen atom. The nitrogen atom contributes one lone pair of electrons to the π system, resulting in a total of six π electrons (4n + 2 where n = 1). Pyrrole is planar, cyclic, and conjugated. Therefore, it fulfills all the criteria for aromaticity and is indeed an aromatic compound.
2. Furan: Another Aromatic Example
Furan is another five-membered heterocycle, but with an oxygen atom instead of nitrogen. Similar to pyrrole, the oxygen atom's lone pair participates in the π system, contributing to the six π electrons. It is planar, cyclic, and conjugated, satisfying all requirements for aromaticity.
3. Thiophene: A Sulfur-Containing Aromatic Compound
Thiophene incorporates a sulfur atom in its five-membered ring. Again, the sulfur atom's lone pair contributes to the π system, leading to the requisite six π electrons. Thiophene's planar, cyclic, and conjugated structure confirms its aromatic nature.
4. Pyridine: A Six-Membered Aromatic Heterocycle
Pyridine is a six-membered heterocycle with a nitrogen atom. However, unlike pyrrole, the nitrogen's lone pair is not part of the π system; it resides in an sp<sup>2</sup> hybrid orbital perpendicular to the ring. The six π electrons from the carbon atoms satisfy Huckel's rule (4n + 2 where n = 1). Pyridine is planar, cyclic, and conjugated, making it aromatic.
5. Imidazole: A Dual Aromatic Nature
Imidazole contains two nitrogen atoms within its five-membered ring. One nitrogen atom contributes a lone pair to the π system, while the other's lone pair remains localized in an sp<sup>2</sup> hybrid orbital. The resulting six π electrons, along with its planar, cyclic, and conjugated structure, make imidazole aromatic.
6. Pyrimidine: Another Six-Membered Aromatic System
Pyrimidine is a six-membered heterocycle with two nitrogen atoms. Both nitrogen atoms' lone pairs are localized, not participating in the π system. Despite this, the remaining six π electrons from the carbon atoms still satisfy Huckel's rule. Its planarity, cyclic nature, and conjugation solidify its aromatic character.
7. Pyran: The Non-Aromatic Heterocycle
Pyran is a six-membered ring containing an oxygen atom. Unlike pyridine, one of the double bonds in pyran is not conjugated with the oxygen lone pair. Therefore, pyran only has four π electrons, failing to satisfy Huckel's rule (4n + 2). Although it is cyclic and largely planar, the lack of complete conjugation prevents it from being aromatic. Therefore, pyran is the heterocycle among the list that is not aromatic.
Exploring Anti-Aromaticity: The Unstable Counterpart
While most of the heterocycles discussed above are aromatic, it's crucial to briefly discuss anti-aromaticity. Anti-aromatic compounds are highly unstable due to having 4n π electrons. They exhibit increased reactivity and possess high energy levels. Cyclobutadiene is a classic example of an anti-aromatic compound.
The Significance of Aromaticity in Chemistry and Beyond
Aromaticity plays a pivotal role in various aspects of chemistry and beyond:
- Drug Design: Many drugs contain aromatic rings, influencing their biological activity and interactions with receptors.
- Material Science: Aromatic compounds are frequently used in the synthesis of polymers and advanced materials with unique properties.
- Organic Synthesis: Understanding aromaticity guides the design and execution of numerous organic reactions.
Conclusion: A Deeper Understanding of Aromatic Heterocycles
This in-depth analysis has highlighted the importance of understanding the criteria for aromaticity and how these criteria apply to various heterocyclic compounds. We've identified pyran as the non-aromatic heterocycle from our selection due to its failure to meet Huckel's rule and possess complete conjugation. The concepts discussed here are fundamental to organic chemistry and have broader implications across diverse scientific fields. By understanding aromaticity, we gain valuable insights into the reactivity, stability, and unique properties of a vast class of organic molecules. Further exploration of the nuances of aromaticity will continue to enrich our understanding of organic chemistry and its applications. This article serves as a foundation for further study into this captivating area of chemistry.
Latest Posts
Latest Posts
-
Which Of The Following Is Bacteriostatic
Mar 29, 2025
-
How Many Unpaired Electrons Does Cobalt Have
Mar 29, 2025
-
Which Of The Following Is Not Output Device
Mar 29, 2025
-
Which Of The Following Bodies Has The Largest Kinetic Energy
Mar 29, 2025
-
A Cell That Contains One Set Of Chromosomes
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Heterocycles Is Not Aromatic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
