How Many Naturally Occurring Elements Are There
News Leon
Mar 16, 2025 · 6 min read
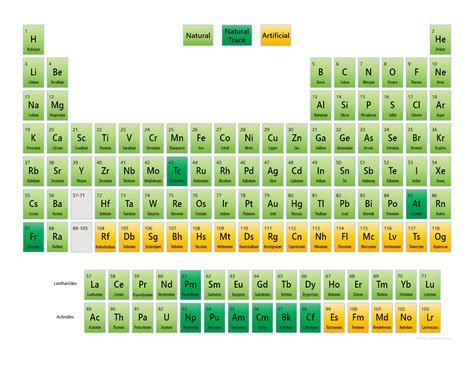
Table of Contents
How Many Naturally Occurring Elements Are There? A Deep Dive into the Periodic Table
The periodic table, that iconic chart adorning countless science classrooms, represents more than just a neatly organized collection of symbols. It's a testament to humanity's understanding of the fundamental building blocks of matter – the chemical elements. But how many of these elements actually exist in nature, untouched by human intervention? The answer, while seemingly straightforward, unveils a fascinating journey through the history of scientific discovery, the intricacies of nuclear physics, and the dynamic processes shaping our planet and the cosmos.
The Count: Naturally Occurring Elements
The straightforward answer is 94. There are 94 naturally occurring chemical elements found on Earth. These elements, ranging from the ubiquitous hydrogen (H) to the heavier, rarer elements like plutonium (Pu), represent the building blocks of everything we see and interact with in our daily lives. They form the basis of rocks, water, air, and even the complex molecules that make up living organisms.
It's crucial to define "naturally occurring" here. This refers to elements found in nature without human intervention through processes like nuclear reactions in reactors or particle accelerators. While scientists have synthesized elements beyond number 94, these are not naturally occurring in the sense that they aren't found in significant quantities in the Earth's crust, atmosphere, or oceans. These artificially produced elements are often highly unstable and decay rapidly.
The Breakdown: Abundance and Distribution
While 94 elements occur naturally, their abundance varies dramatically. Some elements, like oxygen (O) and silicon (Si), are incredibly abundant, forming the vast majority of the Earth's crust. Others, like gold (Au) and platinum (Pt), are exceptionally rare, scattered in trace amounts throughout various geological formations. This distribution isn't uniform; it’s a consequence of complex geological processes, including the formation of the Earth, plate tectonics, volcanic activity, and the constant cycling of materials through the planet's systems.
Abundant Elements: The Building Blocks of Our World
-
Oxygen (O): The most abundant element in the Earth's crust, making up approximately 46% by weight. It's crucial for respiration and is a key component of water and many minerals.
-
Silicon (Si): The second most abundant element, accounting for about 28% of the Earth's crust. It's the primary component of most rocks and minerals.
-
Aluminum (Al): The third most abundant element, forming a significant portion of many clays and other minerals.
-
Iron (Fe): A vital component of the Earth's core and plays a critical role in many biological processes.
-
Calcium (Ca): Essential for bone formation in animals and a key component of many rocks and minerals.
These elements, along with others like sodium (Na), potassium (K), magnesium (Mg), and titanium (Ti), make up the bulk of the Earth's composition. Their abundance reflects the processes involved in the planet's formation and the subsequent geological and chemical transformations it has undergone.
Rare Earth Elements: A Specialized Niche
While abundant elements dominate the Earth's composition, the rarer elements play crucial roles in various technologies and applications. Rare earth elements (REEs), a group of 17 elements including lanthanides and scandium and yttrium, are particularly noteworthy. Despite their name, they're not exceptionally rare in absolute terms, but their dispersed distribution makes extraction and purification challenging. These elements are essential components in many high-tech applications, including magnets, lasers, and catalysts. Their strategic importance has led to ongoing research into sustainable and efficient extraction methods.
The Formation of Elements: Cosmic Forges
The origin of the elements is a story spanning billions of years and encompassing some of the most energetic processes in the universe. The majority of the naturally occurring elements were forged in the hearts of stars.
Stellar Nucleosynthesis: The Birthplace of Elements
Stars are giant nuclear fusion reactors. In their cores, lighter elements like hydrogen (H) and helium (He) are fused together under immense pressure and temperature to create heavier elements. This process, known as stellar nucleosynthesis, is responsible for the creation of most of the elements up to iron (Fe). Heavier elements, beyond iron, are primarily formed during more violent events like supernova explosions.
Supernovae: Cosmic Explosions and Element Creation
Supernovae, the explosive deaths of massive stars, are cataclysmic events that release enormous amounts of energy. These explosions provide the necessary conditions for the creation of elements heavier than iron. The intense neutron flux during a supernova allows for rapid neutron capture, leading to the formation of a wide range of heavier elements, including many of the rare earth elements and elements crucial for life, such as iodine (I) and uranium (U).
Discovering New Elements: A History of Scientific Endeavor
The discovery of new elements has been a continuous process, driving advancements in chemistry and physics. Early discoveries often relied on observing distinctive properties of substances, while later discoveries involved sophisticated techniques like spectroscopy and particle acceleration. While the discovery of naturally occurring elements is largely complete, the quest to understand their behavior and applications continues.
Beyond 94: Synthetic Elements
Scientists have created elements beyond uranium (element 92) through nuclear reactions in particle accelerators. These synthetic elements are highly unstable, with short half-lives, meaning they decay rapidly into other elements. These elements expand our understanding of nuclear physics and the limits of the periodic table, but they aren't considered naturally occurring.
The Significance of Naturally Occurring Elements
The 94 naturally occurring elements are not just a random assortment of atoms; they are intricately linked to the evolution of the universe and the emergence of life on Earth. Their abundance, distribution, and properties have shaped the planet's geology, its atmosphere, and the very building blocks of life itself. Understanding these elements is fundamental to understanding the world around us, from the smallest molecules to the largest geological structures.
Applications and Future Research
The applications of naturally occurring elements are vast and diverse. They are essential for various industries, from construction and manufacturing to medicine and electronics. Ongoing research into these elements focuses on:
-
Sustainable extraction and processing: Developing environmentally friendly methods for extracting and processing elements, particularly rare earth elements.
-
New materials and technologies: Exploring novel applications of elements in advanced materials, energy technologies, and other fields.
-
Understanding elemental cycles: Investigating the flow and transformation of elements in various natural systems, including the Earth's crust, oceans, and atmosphere.
-
Biogeochemical cycling: Examining the roles of trace elements in ecosystems and the impact of human activities on their distribution.
The study of naturally occurring elements remains a vibrant and ever-evolving field, promising new discoveries and applications in the years to come. The 94 elements found in nature represent a remarkable testament to the power of the universe to create complexity and diversity from a relatively small set of fundamental building blocks. Their continued study holds the key to understanding our planet, its history, and its future.
Latest Posts
Latest Posts
-
Which Of The Following Is Polynomial
Mar 17, 2025
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Naturally Occurring Elements Are There . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
