How Many Moles Are In 25 Grams Of Water
News Leon
Mar 28, 2025 · 5 min read
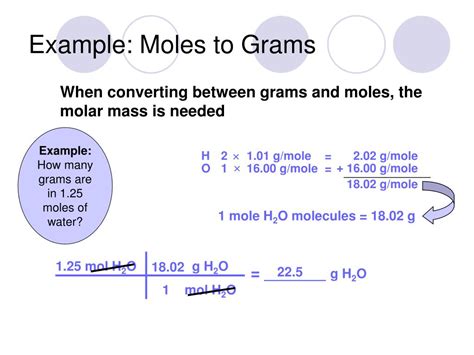
Table of Contents
How Many Moles Are in 25 Grams of Water? A Deep Dive into Moles and Molar Mass
Determining the number of moles in a given mass of a substance is a fundamental concept in chemistry. This article will walk you through the process of calculating the number of moles in 25 grams of water, explaining the underlying principles and providing a step-by-step guide. We'll also explore related concepts like molar mass, Avogadro's number, and the importance of these calculations in various chemical applications.
Understanding Moles: The Chemist's Counting Unit
In chemistry, a mole (mol) isn't a furry creature; it's a unit representing a specific number of particles, whether they are atoms, molecules, ions, or formula units. This number, known as Avogadro's number, is approximately 6.022 x 10<sup>23</sup>. Think of a mole as a chemist's equivalent of a dozen (12) or a gross (144) – a convenient way to count extremely large quantities of tiny particles.
One mole of any substance contains Avogadro's number of particles. This is crucial because it allows chemists to relate the macroscopic world (grams, liters) to the microscopic world (atoms, molecules).
Molar Mass: The Bridge Between Grams and Moles
To convert between grams and moles, we need the molar mass of the substance. Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It's numerically equal to the atomic weight (for elements) or the molecular weight (for compounds) expressed in atomic mass units (amu).
For water (H₂O), we need to calculate its molar mass:
- Hydrogen (H): Atomic weight ≈ 1.008 amu. Since there are two hydrogen atoms in a water molecule, the total contribution from hydrogen is 2 * 1.008 amu = 2.016 amu.
- Oxygen (O): Atomic weight ≈ 16.00 amu. There's one oxygen atom, so its contribution is 16.00 amu.
Therefore, the molecular weight of water is approximately 2.016 amu + 16.00 amu = 18.016 amu. This means the molar mass of water is approximately 18.016 g/mol.
Calculating Moles in 25 Grams of Water: A Step-by-Step Approach
Now, let's calculate the number of moles in 25 grams of water:
1. Identify the known values:
- Mass of water (m) = 25 g
- Molar mass of water (M) = 18.016 g/mol
2. Use the formula:
The formula to convert mass to moles is:
Number of moles (n) = Mass (m) / Molar mass (M)
3. Substitute and solve:
n = 25 g / 18.016 g/mol n ≈ 1.388 moles
Therefore, there are approximately 1.388 moles in 25 grams of water.
Beyond the Calculation: Applications of Mole Calculations
The ability to accurately calculate the number of moles in a given mass is essential in numerous chemical contexts:
-
Stoichiometry: Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Mole calculations are fundamental to predicting the amount of product formed or reactant consumed in a reaction.
-
Solution Chemistry: Concentration is often expressed in molarity (moles of solute per liter of solution). Knowing the number of moles allows for precise preparation of solutions with specific concentrations.
-
Gas Laws: The ideal gas law (PV = nRT) relates pressure (P), volume (V), temperature (T), and the number of moles (n) of a gas. Mole calculations are critical for solving gas law problems.
-
Titrations: Titrations are used to determine the concentration of an unknown solution. Calculations involving moles are crucial for interpreting titration data and determining the unknown concentration.
-
Thermochemistry: Thermochemical calculations often require knowledge of the number of moles of reactants and products to determine the heat released or absorbed during a reaction.
Sources of Error and Precision in Calculations
While our calculation yielded approximately 1.388 moles, it's important to consider potential sources of error:
-
Significant Figures: The precision of our answer is limited by the number of significant figures in our measurements. Using the molar mass of water to four significant figures (18.016 g/mol) suggests that our final answer should also be expressed to four significant figures (1.388 moles). Rounding to fewer significant figures could lead to a less precise result.
-
Purity of Water: Our calculation assumes we're working with pure water. If the water sample contains impurities, the actual number of moles of water present would be slightly lower.
-
Measurement Errors: In a real-world laboratory setting, errors in weighing the water sample can also affect the accuracy of the calculation.
Advanced Concepts: Mole Fractions and Mole Percentages
Beyond simple mole calculations, more sophisticated concepts build upon the understanding of moles:
-
Mole Fraction: The mole fraction of a component in a mixture is the ratio of the number of moles of that component to the total number of moles in the mixture. This is particularly useful when dealing with mixtures of gases or solutions.
-
Mole Percentage: The mole percentage is simply the mole fraction expressed as a percentage.
Conclusion: Moles – The Cornerstone of Chemical Calculations
Understanding moles is paramount for success in chemistry. From stoichiometry to solution preparation, the ability to confidently convert between mass and moles unlocks a deeper understanding of chemical reactions and phenomena. This article provided a comprehensive guide to calculating the number of moles in 25 grams of water and highlighted the broader significance of this fundamental concept within the broader field of chemistry. By mastering these calculations, you're building a strong foundation for more advanced chemical studies and applications. Remember to always pay close attention to significant figures and potential sources of error to ensure the accuracy and precision of your results.
Latest Posts
Latest Posts
-
What Happens To Voltage If Resistance Increases
Mar 31, 2025
-
Calculate The Rotational Inertia Of A Wheel
Mar 31, 2025
-
Equation For The Combustion Of Octane
Mar 31, 2025
-
Of The Halogens Which Has The Smallest Radius
Mar 31, 2025
-
The Following Statements Are Correct Except
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Moles Are In 25 Grams Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
