Equation For The Combustion Of Octane
News Leon
Mar 31, 2025 · 5 min read
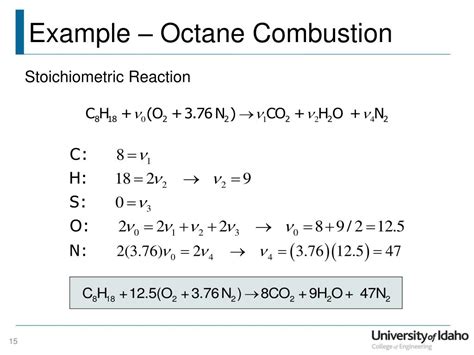
Table of Contents
The Equation for the Combustion of Octane: A Deep Dive into the Chemistry of Fuel
The combustion of octane, a primary component of gasoline, is a fundamental chemical process that powers much of our modern world. Understanding the equation for this reaction, along with its implications and variations, is crucial for anyone interested in chemistry, engineering, or the energy sector. This article will provide a comprehensive exploration of the combustion of octane, covering its balanced equation, stoichiometry, energy considerations, incomplete combustion, and real-world applications.
The Balanced Chemical Equation for Complete Combustion
The complete combustion of octane (C₈H₁₈) involves its reaction with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O). The balanced chemical equation representing this process is:
2C₈H₁₈ + 25O₂ → 16CO₂ + 18H₂O
This equation signifies that two molecules of octane react with 25 molecules of oxygen to yield 16 molecules of carbon dioxide and 18 molecules of water. The equation is balanced because the number of atoms of each element is equal on both sides of the equation. This is crucial because the Law of Conservation of Mass dictates that matter cannot be created or destroyed in a chemical reaction; only rearranged.
Understanding the Stoichiometry
Stoichiometry is the calculation of the relative quantities of reactants and products in chemical reactions. In the combustion of octane, the stoichiometric ratio indicates the precise proportion of octane and oxygen needed for complete combustion. The balanced equation reveals that for every 2 moles of octane, 25 moles of oxygen are required. This ratio is essential in determining the efficiency of combustion engines and optimizing fuel-air mixtures. Any deviation from this ideal ratio will lead to incomplete combustion, as discussed later.
Energy Released During Combustion: Enthalpy Change
The combustion of octane is a highly exothermic reaction, meaning it releases a significant amount of energy in the form of heat. This energy release is responsible for the power generated by internal combustion engines. The enthalpy change (ΔH), often expressed in kilojoules per mole (kJ/mol), represents the amount of heat released or absorbed during a reaction. For the complete combustion of octane, the enthalpy change is a large negative value, typically around -5470 kJ/mol. This negative sign indicates that the reaction releases heat to the surroundings.
Factors Affecting the Energy Released
Several factors can influence the actual amount of energy released during the combustion of octane. These include:
- Temperature: Higher temperatures generally lead to more complete combustion and higher energy release.
- Pressure: Increased pressure can also improve combustion efficiency.
- Presence of Catalysts: While not commonly used in internal combustion engines, catalysts can potentially increase the rate of combustion and energy release.
- Fuel Purity: Impurities in the octane can affect the combustion process and reduce the energy output.
Incomplete Combustion: A Less Efficient Process
Incomplete combustion occurs when there is insufficient oxygen to completely oxidize the octane. This results in the formation of carbon monoxide (CO) and/or soot (carbon, C), in addition to carbon dioxide and water. The equations for incomplete combustion are more complex and can vary depending on the oxygen availability. Some examples include:
- 2C₈H₁₈ + 17O₂ → 16CO + 18H₂O (producing carbon monoxide)
- 2C₈H₁₈ + 9O₂ → 16C + 18H₂O (producing soot)
- C₈H₁₈ + 12.5O₂ → 8CO + 9H₂O + CO₂ (producing a mixture of CO and CO₂)
Incomplete combustion is less efficient than complete combustion because it releases less energy per mole of octane burned. More significantly, it produces harmful pollutants. Carbon monoxide is a highly toxic gas, while soot contributes to air pollution and respiratory problems.
Factors Leading to Incomplete Combustion
Several factors can contribute to incomplete combustion:
- Insufficient Oxygen: The most common cause is a lack of sufficient oxygen to fully oxidize the fuel. This often occurs in poorly tuned engines or when the fuel-air mixture is too rich (too much fuel relative to oxygen).
- Low Temperature: Lower temperatures can slow down the reaction rate and prevent complete oxidation.
- Rapid Combustion: Extremely fast combustion can also prevent complete oxidation, as the oxygen may not have enough time to react with all the fuel molecules.
Real-World Applications and Implications
The combustion of octane is the cornerstone of many technologies and processes:
- Internal Combustion Engines: The primary application is in internal combustion engines powering automobiles, trucks, and other vehicles. The controlled combustion of octane-rich gasoline provides the mechanical energy to drive these engines.
- Power Generation: Octane combustion is also used in some power generation plants, although other fuels like natural gas are more common.
- Industrial Processes: While less common, octane's combustion can provide heat for certain industrial processes.
Environmental Concerns
The combustion of octane, particularly incomplete combustion, contributes significantly to air pollution. The emissions of carbon dioxide are a major contributor to climate change, while carbon monoxide and soot pose serious health risks. Stricter emission regulations and the development of cleaner combustion technologies are crucial to mitigate these environmental concerns. The ongoing research into alternative fuels and engine technologies aims to reduce the reliance on octane-based fuels and lessen their environmental impact.
Advanced Considerations and Further Research
The simple stoichiometric equation provides a good starting point for understanding the combustion of octane, but a more accurate representation requires considering several additional factors. These include:
- Reaction Kinetics: The actual combustion process is a complex sequence of reactions involving free radicals and intermediate species. Understanding the kinetics is essential for optimizing combustion efficiency and minimizing pollutant formation.
- Thermodynamics: Detailed thermodynamic analysis considers the equilibrium constants, entropy changes, and other factors that influence the energy release and product distribution.
- Computational Modeling: Advanced computational fluid dynamics (CFD) models can simulate the combustion process within engines, providing valuable insights into the optimization of combustion chamber design and fuel injection strategies.
- Alternative Fuels: Research into alternative fuels, such as biofuels and hydrogen, is aimed at reducing reliance on fossil fuels like octane and mitigating the environmental impacts of combustion.
Conclusion
The combustion of octane is a complex yet crucial chemical process. Understanding the balanced equation, stoichiometry, energy considerations, and implications of complete and incomplete combustion is vital for addressing challenges related to energy production, environmental protection, and technological advancements. Continued research into optimizing combustion efficiency, reducing emissions, and developing sustainable alternative fuels will remain crucial in the coming years. The journey from a simple chemical equation to a deep understanding of the complexities of octane combustion highlights the power of scientific inquiry and its relevance to our world.
Latest Posts
Related Post
Thank you for visiting our website which covers about Equation For The Combustion Of Octane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
