How Many Lone Pairs Does Nitrogen Have
News Leon
Mar 14, 2025 · 6 min read
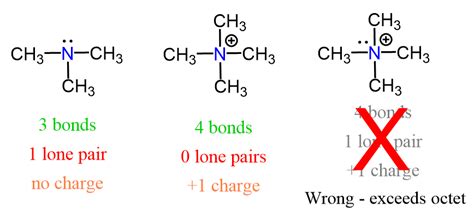
Table of Contents
How Many Lone Pairs Does Nitrogen Have? Understanding Nitrogen's Valence Electrons
Nitrogen, a crucial element in biological molecules and atmospheric gases, possesses a unique electronic structure that governs its reactivity and bonding behavior. Understanding the number of lone pairs on a nitrogen atom is fundamental to grasping its chemical properties and predicting its behavior in various compounds. This comprehensive guide delves into the electron configuration of nitrogen, explains the concept of lone pairs, and explores various examples showcasing nitrogen's bonding patterns.
Nitrogen's Electronic Structure: The Foundation of Lone Pairs
To determine the number of lone pairs on a nitrogen atom, we must first examine its electronic configuration. Nitrogen (N) has an atomic number of 7, meaning it has 7 protons and 7 electrons. These electrons are arranged in energy levels or shells.
Electron Configuration and Valence Electrons
The electronic configuration of nitrogen is 1s²2s²2p³. This means:
- 1s²: Two electrons occupy the lowest energy level (1s orbital).
- 2s²: Two electrons occupy the next energy level (2s orbital).
- 2p³: Three electrons occupy the higher energy p orbitals (2p<sub>x</sub>, 2p<sub>y</sub>, 2p<sub>z</sub>).
The valence electrons are the electrons in the outermost energy level, which are involved in chemical bonding. For nitrogen, these are the five electrons in the second energy level (2s²2p³). These valence electrons determine nitrogen's reactivity and bonding capacity.
Understanding Lone Pairs: Non-bonding Electron Pairs
A lone pair, also known as a non-bonding pair of electrons, is a pair of valence electrons that is not involved in covalent bonding. These electrons are localized on the atom and contribute to its overall electronic structure and properties. They influence the molecule's shape, polarity, and reactivity.
Determining the Number of Lone Pairs on Nitrogen
Nitrogen has five valence electrons. To achieve a stable octet (eight electrons in its valence shell), it typically forms three covalent bonds, sharing three of its valence electrons. This leaves two electrons, forming one lone pair.
Therefore, a nitrogen atom typically has one lone pair of electrons.
Visualizing Nitrogen's Lone Pair: Lewis Structures
Lewis structures, also known as Lewis dot diagrams, are a simplified representation of an atom's valence electrons and their involvement in bonding. They are particularly useful for visualizing lone pairs. In a Lewis structure for nitrogen, the five valence electrons are represented as dots around the nitrogen symbol (N). Three dots are involved in bonding, and the remaining two form the lone pair.
For example, in ammonia (NH₃), the nitrogen atom forms three covalent bonds with three hydrogen atoms, using three of its five valence electrons. The remaining two electrons form a lone pair on the nitrogen atom.
H
|
H - N - H
|
Lone Pair
Nitrogen's Bonding in Different Compounds: Examples
Nitrogen's ability to form one lone pair and three covalent bonds leads to its participation in a wide variety of compounds. Let's look at some examples:
1. Ammonia (NH₃)
As mentioned earlier, in ammonia, nitrogen forms three covalent bonds with hydrogen atoms, leaving one lone pair. This lone pair is responsible for ammonia's basicity; it can readily accept a proton (H⁺), forming the ammonium ion (NH₄⁺).
2. Amines (R₃N)
Amines are organic compounds derived from ammonia, where one or more hydrogen atoms are replaced by alkyl or aryl groups (R). These amines also possess a nitrogen atom with one lone pair, contributing to their basic nature. Similar to ammonia, amines can act as bases by accepting protons.
3. Amides (RCONH₂)
Amides are organic compounds containing a carbonyl group (C=O) bonded to a nitrogen atom. The nitrogen atom in amides still possesses a lone pair, although its basicity is reduced compared to ammonia or amines due to the electron-withdrawing effect of the carbonyl group.
4. Nitriles (RC≡N)
Nitriles contain a carbon-nitrogen triple bond (C≡N). Although the nitrogen atom in a nitrile appears to have only one lone pair in the simplified Lewis structure, it is more accurately depicted as having multiple resonance structures, each with a different distribution of electrons and less clearly defined lone pairs.
5. Nitrous Oxide (N₂O)
Nitrous oxide, also known as laughing gas, has a more complex structure. While one nitrogen atom may appear to have a lone pair based on formal charge, the actual distribution of electrons across resonance structures leads to a less clear identification of traditional lone pairs. The bonding in N₂O involves multiple bonds and resonance, obscuring a simple lone pair count.
6. Nitrate Ion (NO₃⁻)
In the nitrate ion, the nitrogen atom is surrounded by three oxygen atoms, with a formal charge of +1. There are no lone pairs on the nitrogen; however, the oxygen atoms each possess lone pairs. The delocalized electrons within the nitrate ion's resonance structures obscure the concept of a singular lone pair on the nitrogen.
Exceptions and Complex Cases: Beyond the Simple Lone Pair
While the general rule is that nitrogen has one lone pair, there are exceptions and cases where the concept of lone pairs becomes less straightforward. These include:
- Compounds with multiple bonds: In compounds with multiple bonds involving nitrogen, like nitriles, the electron distribution becomes more complex, and the lone pair is not as clearly defined.
- Resonance structures: In molecules with resonance structures, the electron distribution is delocalized, making it difficult to assign lone pairs to specific atoms.
- Formal charges: Formal charges can sometimes mask the presence of lone pairs or make them difficult to directly visualize.
The Significance of Nitrogen's Lone Pair: Impact on Properties and Reactivity
The presence of a lone pair on nitrogen significantly influences its chemical properties and reactivity.
- Basicity: The lone pair acts as a Lewis base, readily donating electrons to form coordinate covalent bonds with Lewis acids (electron acceptors). This explains the basic nature of ammonia and amines.
- Hydrogen bonding: The lone pair on nitrogen allows it to participate in hydrogen bonding, influencing the properties of many nitrogen-containing compounds. Hydrogen bonding contributes to the high boiling points of ammonia and water.
- Coordination chemistry: The lone pair makes nitrogen a good ligand in coordination complexes, forming bonds with metal ions.
- Reactivity: The lone pair's availability dictates nitrogen's reactivity. It can participate in reactions like alkylation, acylation, and protonation.
Conclusion: A Deeper Understanding of Nitrogen's Chemistry
In conclusion, understanding the number of lone pairs on a nitrogen atom is crucial for comprehending its bonding behavior and chemical properties. While a nitrogen atom generally possesses one lone pair, this simple picture becomes more complex in molecules with multiple bonds or resonance structures. The presence and behavior of this lone pair are key to nitrogen's widespread presence and importance in organic and inorganic chemistry, significantly impacting the properties and reactivity of countless compounds. By understanding these principles, we can better predict and explain the chemical behavior of nitrogen in diverse applications.
Latest Posts
Latest Posts
-
How Is A Conductor Different From An Insulator
Mar 14, 2025
-
Moment Of Inertia Of Spherical Shell
Mar 14, 2025
-
The Last Leaf By O Henry Summary
Mar 14, 2025
-
Why Is The Demand Curve Downward Sloping
Mar 14, 2025
-
The Embryo Sac Of An Angiosperm Contains
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Lone Pairs Does Nitrogen Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
