How Is A Conductor Different From An Insulator
News Leon
Mar 14, 2025 · 6 min read
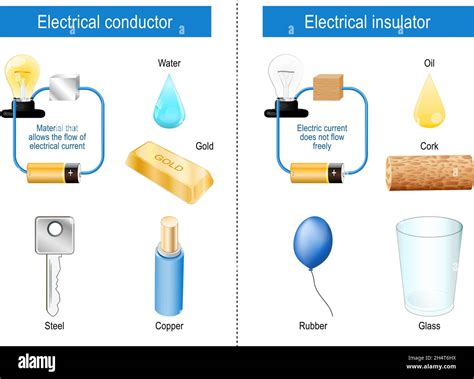
Table of Contents
How is a Conductor Different From an Insulator? A Deep Dive into Electrical Properties
The world of electricity is governed by the fundamental interaction between two key types of materials: conductors and insulators. Understanding their differences is crucial not only for comprehending basic electrical principles but also for designing and using countless electrical devices safely and effectively, from simple circuits to complex power grids. This article will delve deep into the contrasting properties of conductors and insulators, exploring their atomic structures, practical applications, and the subtle nuances that differentiate them.
The Atomic Dance: Understanding Conduction and Insulation
The key to understanding the difference between conductors and insulators lies at the atomic level. The behavior of electrons, specifically their mobility within the material, determines whether a substance will readily conduct or resist the flow of electric current.
Conductors: The Free Electron Highway
Conductors are materials that allow electrons to flow freely through them. This freedom of movement is directly linked to their atomic structure. In conductors, the outermost electrons, known as valence electrons, are loosely bound to their atoms. This loose binding allows these electrons to easily detach from their parent atoms and become delocalized, forming a "sea" of freely moving electrons. When an electric field is applied, these free electrons are readily accelerated, creating an electric current.
Examples of excellent conductors include:
- Metals: Copper, silver, gold, aluminum, and iron are prime examples. Their metallic bonding, characterized by a lattice of positively charged ions surrounded by a sea of delocalized electrons, contributes to their exceptional conductivity.
- Electrolytes: These are solutions containing ions that are free to move, such as saltwater or molten salts. The movement of these charged ions constitutes an electric current.
- Plasma: A highly ionized gas where electrons are stripped from atoms, resulting in a highly conductive state. This is seen in things like lightning or fluorescent lights.
Insulators: The Electron Lockdown
Insulators, in stark contrast to conductors, strongly resist the flow of electric current. This resistance stems from the tight binding of electrons to their atoms. In insulators, valence electrons are tightly held and are not easily freed to move around. Even under the influence of an electric field, these electrons remain largely confined to their respective atoms, preventing the formation of a significant electric current.
Common examples of insulators include:
- Rubber: Widely used in electrical applications due to its high resistance to current flow.
- Plastics: Such as PVC, Teflon, and polyethylene, are excellent insulators found in many electrical components and housings.
- Glass: A non-conductive material with high dielectric strength, used in various insulating applications.
- Wood: Depending on moisture content, wood can exhibit insulating properties, although it's less reliable than synthetic materials.
- Ceramics: Many ceramic materials are excellent insulators due to their strong ionic bonds.
Beyond the Basics: Exploring Key Differences
While the free electron mobility is the fundamental difference, several other key properties distinguish conductors from insulators:
1. Resistivity: A Measure of Resistance
Resistivity is a measure of a material's opposition to the flow of electric current. Conductors have very low resistivity, allowing current to flow easily. Insulators, on the other hand, exhibit very high resistivity, significantly hindering current flow.
2. Conductivity: The Inverse of Resistivity
Conductivity is the reciprocal of resistivity, representing the ease with which a material allows current to flow. Conductors possess high conductivity, while insulators have very low conductivity.
3. Energy Band Gap: The Electron's Energy Landscape
The energy band gap is the energy difference between the valence band (where electrons are normally located) and the conduction band (where electrons can move freely). In conductors, the valence and conduction bands overlap, allowing electrons to easily transition to the conduction band. In insulators, the energy band gap is large, requiring a significant amount of energy to excite electrons to the conduction band. This energy barrier prevents the free movement of electrons.
4. Temperature Dependence: The Heat Factor
The conductivity of conductors generally decreases with increasing temperature. As temperature rises, the increased atomic vibrations interfere with the flow of electrons. Insulators, on the other hand, may exhibit a slight increase in conductivity at higher temperatures as some electrons gain enough energy to overcome the band gap. However, this increase is typically small and doesn't significantly alter their insulating properties.
5. Applications: Harnessing the Contrast
The contrasting properties of conductors and insulators are exploited in countless applications.
Conductors find use in:
- Wires and Cables: For transmitting electricity.
- Electrical Components: Such as resistors (though these often involve controlling electron flow), capacitors, and inductors.
- Electronic Circuits: Forming pathways for current flow in integrated circuits.
- Power Transmission Lines: For carrying large amounts of electricity over long distances.
Insulators are crucial in:
- Electrical Insulation: Preventing short circuits and electric shocks.
- Coating Wires and Cables: Providing a protective layer against environmental factors and accidental contact.
- Circuit Boards: Separating conductive pathways and preventing shorts.
- High-Voltage Equipment: Protecting personnel and equipment from dangerous voltages.
Semiconductors: Bridging the Gap
While conductors and insulators represent distinct extremes, there's a fascinating intermediate category called semiconductors. Semiconductors have electrical conductivity that lies between that of conductors and insulators. Their conductivity can be significantly altered by factors like temperature, doping (introducing impurities), or light exposure. This unique property makes semiconductors the foundation of modern electronics, allowing for the creation of transistors, diodes, and integrated circuits.
The behavior of semiconductors is largely governed by their energy band gap. Semiconductors possess a smaller band gap than insulators, meaning that electrons can be excited to the conduction band with relatively less energy. This allows for the controlled flow of electrons, enabling the intricate functionality of semiconductor devices. Silicon and germanium are two of the most common semiconductor materials.
Beyond the Binary: Considering Other Factors
While the fundamental difference between conductors and insulators lies in electron mobility, other factors can influence their behavior:
- Purity: Impurities in conductors can increase their resistivity. Similarly, impurities in insulators can decrease their resistance.
- Temperature: While generally affecting conductivity in a predictable way, extreme temperature conditions can alter material properties significantly.
- Frequency: At high frequencies, the behavior of both conductors and insulators can change due to skin effect (current concentrating at the surface of a conductor) and dielectric losses (energy dissipation in insulators).
- Moisture: Moisture can dramatically reduce the insulating properties of many materials, creating conductive pathways.
Conclusion: A Continuing Exploration
The distinction between conductors and insulators, rooted in the fundamental behavior of electrons within atomic structures, is crucial to our understanding and utilization of electricity. While seemingly simple, the interplay of conductivity, resistivity, and other factors reveals a complex world of electrical properties, leading to countless applications and technological advancements. The continuing exploration of these material properties promises further innovations in the fields of electronics, energy, and beyond. From the simplest light switch to the most complex supercomputer, the carefully controlled interplay between conductors and insulators underpins our modern technological world.
Latest Posts
Latest Posts
-
Convert Timestamp To Date Time Python
Mar 14, 2025
-
Difference Between A Monologue And A Soliloquy
Mar 14, 2025
-
Coordination Number Of Body Centered Cubic
Mar 14, 2025
-
Integral Of 1 X 2 3
Mar 14, 2025
-
How Many Cubic Centimeters In 1 Cubic Meter
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Is A Conductor Different From An Insulator . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
