How Many Hydrogen Bonds Does Guanine And Cytosine Have
News Leon
Mar 21, 2025 · 6 min read
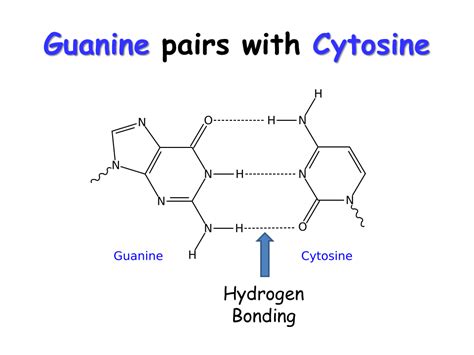
Table of Contents
How Many Hydrogen Bonds Do Guanine and Cytosine Have? A Deep Dive into Base Pairing
The elegance of DNA's double helix structure hinges on the precise pairing of its constituent nitrogenous bases. Understanding the nature of these interactions is crucial to grasping the mechanisms of DNA replication, transcription, and overall genetic stability. This article delves deep into the hydrogen bonding between guanine (G) and cytosine (C), explaining not only the number of bonds but also the intricacies of their formation, implications for DNA structure, and the broader context within molecular biology.
The Fundamentals of Hydrogen Bonding
Before focusing on the G-C interaction, it's essential to understand the basic principles of hydrogen bonding. Hydrogen bonds are a special type of dipole-dipole attraction between molecules, not a covalent bond. They occur when a hydrogen atom bonded to a highly electronegative atom (like oxygen or nitrogen) is attracted to another electronegative atom in a different molecule. This attraction is significantly weaker than a covalent bond but plays a crucial role in determining the structure and properties of many biological molecules, including DNA. The strength of a hydrogen bond depends on several factors, including the electronegativity of the atoms involved and the geometry of the interaction.
Key Features of Hydrogen Bonds:
- Electrostatic Attraction: The primary driving force is the electrostatic attraction between the partially positive hydrogen atom (δ+) and the partially negative electronegative atom (δ-).
- Directional: Hydrogen bonds are directional, meaning they are strongest when the atoms are aligned linearly.
- Weak but Cumulative: While individual hydrogen bonds are relatively weak, the cumulative effect of many hydrogen bonds can be substantial, leading to significant stabilization of biological structures.
Guanine-Cytosine Base Pairing: Three Hydrogen Bonds
The key answer to our central question is: Guanine and cytosine form three hydrogen bonds. This is in contrast to adenine (A) and thymine (T) which form only two hydrogen bonds. These three hydrogen bonds contribute to the stability of the DNA double helix. Let's examine these bonds in detail:
The Three Hydrogen Bonds in Detail:
-
Bond 1: A hydrogen bond forms between the amino group (-NH₂) on cytosine and the carbonyl group (=O) on guanine. The hydrogen atom from the amino group of cytosine is attracted to the oxygen atom of the carbonyl group on guanine.
-
Bond 2: A hydrogen bond forms between the nitrogen atom (N) in the ring structure of cytosine and the amino group (-NH₂) attached to the ring structure of guanine. The hydrogen from the guanine amino group is attracted to the nitrogen in the cytosine ring.
-
Bond 3: A hydrogen bond forms between the carbonyl group (=O) on cytosine and the amino group (-NH₂) on guanine. The hydrogen from the amino group of guanine is attracted to the oxygen atom of the carbonyl group on cytosine.
These three hydrogen bonds are precisely arranged to create a stable and geometrically compatible base pair. The specific arrangement of donor and acceptor atoms contributes to the specificity of base pairing in DNA. Incorrect pairings are less energetically favorable due to steric hindrance and the lack of optimal hydrogen bond formation.
The Significance of Three Hydrogen Bonds in G-C Base Pairing
The presence of three hydrogen bonds between guanine and cytosine has significant implications for DNA structure and function:
-
Increased Stability: The three hydrogen bonds contribute to a higher melting temperature (Tm) of DNA containing a higher G-C content. This is because more energy is required to break three hydrogen bonds compared to two. Organisms inhabiting extreme environments often have DNA with higher G-C content for increased stability.
-
Influence on DNA Structure: The stronger G-C base pairs influence the overall shape and rigidity of the DNA double helix. Regions rich in G-C base pairs tend to be more tightly packed.
-
Role in Replication and Transcription: The precise formation and breaking of hydrogen bonds are crucial for DNA replication and transcription. DNA polymerase accurately recognizes and forms new base pairs during replication, relying on the specificity of hydrogen bonding. Similarly, RNA polymerase relies on these interactions during transcription.
-
DNA stability in various conditions: The stronger G-C bonds provide higher resilience to extreme conditions such as high temperature or pH variations. This is critical in the survival of extremophiles.
Comparison with Adenine-Thymine Base Pairing
It's useful to compare the G-C base pair with the A-T base pair, which forms only two hydrogen bonds. This difference in the number of hydrogen bonds explains why DNA with a higher G-C content is generally more stable than DNA with a higher A-T content. The increased stability is particularly important in regions of the DNA that need to resist denaturation or maintain their structure under stress.
Key Differences:
| Feature | Guanine-Cytosine (G-C) | Adenine-Thymine (A-T) |
|---|---|---|
| Number of Bonds | Three | Two |
| Bond Strength | Stronger | Weaker |
| Melting Point | Higher | Lower |
| Stability | Higher | Lower |
Beyond Base Pairing: The Broader Biological Context
The understanding of G-C base pairing extends far beyond its role in DNA structure. It's integral to various biological processes and phenomena:
-
Genetic Code: The precise sequence of G, C, A, and T dictates the genetic code and ultimately the amino acid sequence of proteins.
-
Gene Regulation: The presence of G-C rich regions can influence gene expression by affecting the binding of regulatory proteins.
-
Evolutionary Significance: The stability conferred by G-C base pairing plays a role in the evolution and adaptation of organisms to various environments.
-
Medical Applications: Understanding base pairing is crucial in fields like genetic engineering, gene therapy, and the development of diagnostic tools.
Potential for Errors and Repair Mechanisms
Despite the robust nature of hydrogen bonding, errors in base pairing can occur during DNA replication. These errors, called mutations, can have significant consequences. However, cells possess sophisticated repair mechanisms to detect and correct such errors, ensuring the fidelity of genetic information. These repair mechanisms involve enzymes that recognize mismatched base pairs and either remove or correct them.
Conclusion
The number of hydrogen bonds between guanine and cytosine—three—is not merely a detail; it's a fundamental aspect of molecular biology with wide-ranging implications. The strength and specificity of these bonds underpin the stability of the DNA double helix, influence gene expression, and contribute to the fidelity of genetic information. Understanding the nuances of G-C base pairing is essential for comprehending the intricacies of life itself. Further research continues to refine our knowledge of this critical interaction, unveiling more details about its role in various biological processes and its contribution to the remarkable complexity of life.
Latest Posts
Latest Posts
-
Ch3 Ch2 Ch Ch Ch2 Ch2 Ch3 Name
Mar 22, 2025
-
Which Of The Following Best Describes A Population
Mar 22, 2025
-
Energy Level Diagram Of Hydrogen Atom
Mar 22, 2025
-
Red Blood Cells Put In A Hypotonic Solution Will
Mar 22, 2025
-
Compound A Forms A Red Orange Precipitate
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Many Hydrogen Bonds Does Guanine And Cytosine Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
