How Many Electrons Are In The Outer Shell Of Carbon
News Leon
Apr 06, 2025 · 5 min read
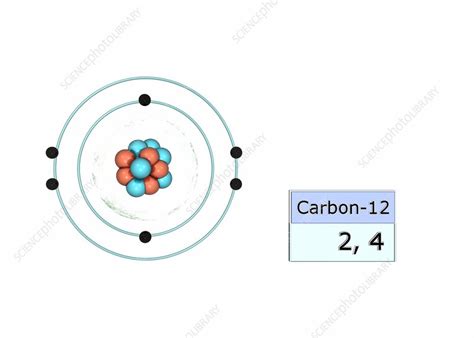
Table of Contents
How Many Electrons Are in the Outer Shell of Carbon? Understanding Carbon's Valence Electrons
Carbon, the backbone of life as we know it, holds a unique position in the periodic table. Its properties, largely determined by its electron configuration, are crucial to its ability to form a vast array of molecules, from simple organic compounds to complex biomolecules like DNA. A key aspect of understanding carbon's reactivity and bonding capabilities lies in knowing how many electrons are in its outer shell. This article will delve deep into this question, exploring the electron configuration of carbon, its valence electrons, and the implications for its chemical behavior.
Understanding Electron Shells and Valence Electrons
Before focusing specifically on carbon, let's establish a foundational understanding of electron shells and valence electrons. Atoms are composed of a nucleus containing protons and neutrons, surrounded by electrons orbiting in distinct energy levels called shells or electron shells. These shells can hold a specific number of electrons; the closer a shell is to the nucleus, the lower its energy level and the fewer electrons it can hold.
The first shell, closest to the nucleus, can hold a maximum of two electrons. The second shell can hold up to eight electrons. The third shell can accommodate up to 18 electrons, and so on. The pattern continues, with each subsequent shell having a higher capacity for electrons.
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound to the nucleus and are, therefore, the ones most likely to participate in chemical bonding with other atoms. The number of valence electrons determines an element's chemical reactivity and the types of bonds it can form.
Carbon's Electron Configuration: Unveiling the Outer Shell
Carbon's atomic number is 6, meaning it has six protons in its nucleus and six electrons orbiting around it. To determine the electron configuration, we fill the electron shells according to the Aufbau principle, which dictates that electrons occupy the lowest available energy levels first.
Therefore, carbon's electron configuration is 1s²2s²2p². Let's break this down:
- 1s²: Two electrons fill the first electron shell (n=1). The 's' subshell can hold a maximum of two electrons.
- 2s²: Two electrons fill the 2s subshell of the second electron shell (n=2).
- 2p²: Two electrons fill the 2p subshell of the second electron shell. The 'p' subshell can hold up to six electrons, but in carbon, only two occupy it.
This electron configuration clearly shows that carbon has four electrons in its outermost shell (the second shell). These four electrons are carbon's valence electrons.
The Significance of Four Valence Electrons
The presence of four valence electrons is crucial to carbon's remarkable versatility in forming chemical bonds. Carbon can achieve a stable electron configuration by either gaining four electrons (highly unlikely due to the high energy required) or sharing its four valence electrons with other atoms through covalent bonds. This ability to form four covalent bonds allows carbon to create a vast array of complex molecules with diverse structures and functionalities.
Carbon's Bonding Prowess: A Consequence of its Outer Shell Electrons
Carbon's four valence electrons enable it to form strong covalent bonds with a wide range of elements, including hydrogen, oxygen, nitrogen, sulfur, and other carbon atoms. This capacity for diverse bonding is at the heart of organic chemistry and the basis for the incredible diversity of life's building blocks.
Here are some examples illustrating carbon's bonding versatility:
-
Carbon-Carbon Bonds: Carbon atoms can bond readily with other carbon atoms, forming long chains, branched structures, and rings. This ability is fundamental to the formation of large, complex organic molecules like polymers and macromolecules. The different types of carbon-carbon bonds (single, double, and triple) also contribute to the vast structural diversity of organic compounds.
-
Carbon-Hydrogen Bonds: Carbon forms exceptionally strong bonds with hydrogen atoms, which are abundant in nature. These bonds are prevalent in organic molecules, including hydrocarbons (like methane and ethane) and many organic functional groups.
-
Carbon-Oxygen Bonds: Carbon's bonds with oxygen are critical in many biological molecules. Carbon-oxygen double bonds are found in carbonyl groups (aldehydes, ketones), carboxylic acids, and esters, which play essential roles in biological processes.
-
Carbon-Nitrogen Bonds: The carbon-nitrogen bond is also important in organic and biological chemistry. It's found in amino acids, the building blocks of proteins, and in nitrogenous bases of DNA and RNA.
The strength and stability of these covalent bonds, arising from the sharing of electrons between carbon and other atoms, are responsible for the diversity and complexity of carbon-based molecules.
Implications for Carbon's Chemical and Biological Roles
The presence of four valence electrons in carbon's outer shell has profound implications for its role in chemistry and biology:
-
Organic Chemistry's Foundation: The unique bonding capabilities of carbon underpin the vast field of organic chemistry. The ability to form long chains, branched structures, rings, and multiple bonds gives rise to millions of organic compounds, forming the basis of countless materials, pharmaceuticals, and biological molecules.
-
Biological Macromolecules: Carbon is the central element in the four major classes of biological macromolecules: carbohydrates, lipids, proteins, and nucleic acids. These molecules are essential for life, providing energy, structure, catalysis, and genetic information storage.
-
Material Science Applications: Carbon's versatility extends to materials science, where it finds applications in various materials like graphite (used in pencils and lubricants) and diamond (one of the hardest materials known). Carbon nanotubes and graphene, advanced carbon-based materials, show potential in numerous technological applications.
-
Environmental Significance: Carbon's role in the environment is equally important. The carbon cycle, which involves the exchange of carbon between the atmosphere, oceans, and living organisms, is vital for regulating Earth's climate and supporting life.
Conclusion: The Four Valence Electrons – Key to Carbon's Uniqueness
In summary, carbon's outer shell contains four valence electrons. This seemingly simple fact is the foundation for carbon's exceptional chemical and biological significance. Its ability to form four strong covalent bonds with a wide range of atoms allows for the creation of an incredibly diverse range of molecules, from simple organic compounds to complex biomolecules and advanced materials. Understanding the electron configuration of carbon and the implications of its four valence electrons is essential for grasping the fundamental principles of chemistry, biology, and materials science. This knowledge underscores why carbon is indeed the building block of life and a cornerstone of countless technological advancements.
Latest Posts
Latest Posts
-
What Is The Lcm Of 4 5 And 6
Apr 07, 2025
-
H2so4 Ionic Or Molecular Acid Or Base
Apr 07, 2025
-
Evaluate 3 To The Power Of 4
Apr 07, 2025
-
Does Bf3 Have A Net Dipole Moment
Apr 07, 2025
-
What Happens When Two Forces Act In The Same Direction
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In The Outer Shell Of Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
