H2so4 Ionic Or Molecular Acid Or Base
News Leon
Apr 07, 2025 · 5 min read
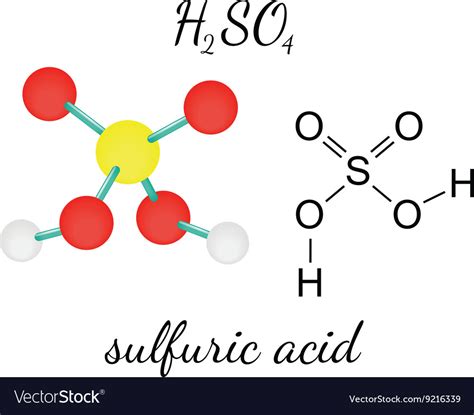
Table of Contents
H₂SO₄: Ionic or Molecular Acid? Delving into the Nature of Sulfuric Acid
Sulfuric acid (H₂SO₄), a cornerstone chemical in countless industrial processes, often sparks confusion regarding its classification as either an ionic or molecular compound. The answer, as with many things in chemistry, isn't a simple yes or no. Understanding the true nature of H₂SO₄ requires examining its behavior in different contexts and appreciating the nuances of chemical bonding. This comprehensive article will explore the complexities of sulfuric acid's structure and its implications for its classification.
The Molecular Perspective: Covalent Bonds and Structure
At its core, sulfuric acid is a molecular compound. This is evident from its structure, which is based on covalent bonds. The sulfur atom (S) is centrally located and forms covalent bonds with four oxygen atoms (O). Two of these oxygen atoms are also bonded to hydrogen atoms (H), forming hydroxyl (-OH) groups. This arrangement gives sulfuric acid its characteristic tetrahedral geometry around the sulfur atom.
Understanding Covalent Bonding in H₂SO₄
Covalent bonding occurs when atoms share electrons to achieve a stable electron configuration. In H₂SO₄, the sulfur atom shares electrons with the oxygen atoms, and the oxygen atoms share electrons with the hydrogen atoms. This sharing of electrons creates strong covalent bonds that hold the molecule together. The strength of these bonds is reflected in the high boiling point of sulfuric acid (337°C), significantly higher than many purely ionic compounds.
The Role of Electronegativity
The electronegativity difference between sulfur and oxygen plays a crucial role. Oxygen is significantly more electronegative than sulfur, meaning it attracts the shared electrons more strongly. This leads to a polarization of the S-O bonds, with a partial negative charge (δ-) on the oxygen atoms and a partial positive charge (δ+) on the sulfur atom. This polarity contributes to the acid's reactivity.
The Ionic Perspective: Dissociation in Aqueous Solutions
Despite its fundamentally molecular nature, sulfuric acid exhibits ionic characteristics when dissolved in water. This is because the highly polar S-O bonds and the relatively weak O-H bonds facilitate the dissociation of H⁺ ions.
The First Dissociation: A Strong Acid Behavior
When H₂SO₄ is added to water, it readily donates its first proton (H⁺) to a water molecule, forming the hydronium ion (H₃O⁺) and the bisulfate ion (HSO₄⁻). This first dissociation is considered complete, or nearly so, making sulfuric acid a strong acid with respect to its first proton. The equilibrium strongly favors the formation of ions.
H₂SO₄(aq) + H₂O(l) → H₃O⁺(aq) + HSO₄⁻(aq)
The Second Dissociation: A Weaker Acid Behavior
The bisulfate ion (HSO₄⁻) can also donate a proton, but to a much lesser extent. This second dissociation is significantly weaker, meaning the equilibrium lies more towards the undissociated HSO₄⁻. This makes sulfuric acid a weak acid with respect to its second proton.
HSO₄⁻(aq) + H₂O(l) ⇌ H₃O⁺(aq) + SO₄²⁻(aq)
The Importance of the Solvent
The behavior of sulfuric acid is heavily dependent on the solvent. In water, the strong polar interactions facilitate dissociation into ions, thus showcasing its ionic properties. However, in nonpolar solvents, the dissociation is significantly suppressed, and the molecular nature of H₂SO₄ becomes more prominent.
The Amphoteric Nature: A Subtlety to Consider
While primarily recognized as an acid, sulfuric acid exhibits subtle amphoteric behavior. This means it can act as both an acid and a base, depending on the reaction conditions.
Sulfuric Acid as an Acid: The Dominant Behavior
As discussed earlier, sulfuric acid readily donates protons, behaving as an acid in most reactions. This is its dominant characteristic.
Sulfuric Acid as a Base: A Less Common Role
In the presence of a very strong acid, sulfuric acid can act as a base, accepting a proton. This behavior is less common but highlights its amphoteric nature. For example, with superacids like fluorosulfuric acid (FSO₃H), sulfuric acid can act as a base.
Why the Confusion Persists: A Matter of Perspective
The ongoing debate about whether H₂SO₄ is ionic or molecular arises from the fact that it exhibits properties characteristic of both. Its structure is undeniably molecular, with covalent bonds holding the atoms together. However, in aqueous solutions, it undergoes significant dissociation, producing ions and behaving like an ionic compound. The key is to acknowledge that its behavior is context-dependent.
Applications Leveraging its Dual Nature
The versatility of sulfuric acid stems from its ability to act as both a molecular compound and a source of ions. This duality is exploited in numerous applications:
- Industrial Processes: In many industrial processes, the high concentration of H⁺ ions drives chemical reactions, making sulfuric acid an effective catalyst and reagent.
- Battery Manufacturing: Sulfuric acid's ionic nature is crucial for its role as the electrolyte in lead-acid batteries, allowing for the flow of ions and the generation of electricity.
- Dehydrating Agent: The strong affinity of sulfuric acid for water (its molecular property) makes it an excellent dehydrating agent, used to remove water from substances.
- Mineral Processing: In mining, sulfuric acid is used to leach out valuable metals from ores, utilizing both its acidic and ionic properties.
Conclusion: A Balanced Perspective
Sulfuric acid's classification as ionic or molecular isn't a binary choice. Its true nature is a blend of both, a testament to the complexity of chemical bonding and reactivity. Understanding this duality is essential for appreciating the wide range of applications where this versatile compound plays a critical role. It's vital to consider the context – in its pure form, it's molecular; in aqueous solution, its ionic character dominates. This understanding allows for a more nuanced and complete appreciation of sulfuric acid's importance in chemistry and industry. The key is to embrace the multifaceted nature of this remarkable compound rather than trying to force it into a rigid classification.
Latest Posts
Latest Posts
-
What Is The Si Unit Of Current
Apr 09, 2025
-
The Electric Flux Through The Shaded Surface Is
Apr 09, 2025
-
Balanced Equation Of Nacl And Agno3
Apr 09, 2025
-
How Many Electrons Can Occupy The 3d Subshell
Apr 09, 2025
-
Difference Between And Enzyme And A Hormone
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about H2so4 Ionic Or Molecular Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.