How Many Atoms Are In A Fcc Unit Cell
News Leon
Mar 25, 2025 · 6 min read
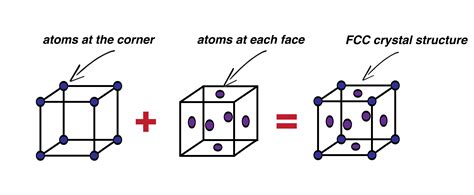
Table of Contents
How Many Atoms Are in an FCC Unit Cell? A Deep Dive into Crystallography
Understanding crystal structures is fundamental to materials science, chemistry, and physics. One of the most common crystal structures is the face-centered cubic (FCC) structure. But a question often arises: how many atoms are actually within an FCC unit cell? The answer isn't immediately obvious, and requires a closer look at the geometry and atom positions within the unit cell. This article will delve into the intricacies of FCC structures, explaining precisely how to calculate the number of atoms, and exploring the implications of this calculation for understanding material properties.
Understanding the Face-Centered Cubic (FCC) Structure
Before we tackle the atom count, let's establish a solid understanding of what an FCC unit cell actually is. An FCC unit cell is a type of cubic crystal structure where atoms are located at each of the eight corners of a cube, and also at the center of each of the six faces. This arrangement leads to a highly efficient packing arrangement, maximizing the use of space and contributing to the properties of many common metals.
Atom Positions in the FCC Unit Cell
The crucial aspect to remember is that atoms in a crystal lattice aren't exclusively confined within the boundaries of a single unit cell. Atoms are shared between adjacent unit cells. This sharing significantly affects the number of atoms we can definitively attribute to a single unit cell.
-
Corner Atoms: Each corner atom is shared by eight adjacent unit cells. Therefore, only 1/8th of each corner atom belongs to a single unit cell. Since there are eight corners, this contributes (8 corners * 1/8 atom/corner) = 1 atom.
-
Face-Centered Atoms: Each atom located at the center of a face is shared by two adjacent unit cells. Thus, only 1/2 of each face-centered atom belongs to a single unit cell. With six faces, this contributes (6 faces * 1/2 atom/face) = 3 atoms.
Calculating the Total Number of Atoms
Adding the contributions from both the corner and face-centered atoms, we arrive at the total number of atoms within a single FCC unit cell:
1 atom (from corners) + 3 atoms (from faces) = 4 atoms
Therefore, there are four atoms in a single FCC unit cell. This seemingly simple calculation has profound implications for understanding the properties of materials that crystallize in the FCC structure.
Implications of the Four Atoms per Unit Cell
The fact that there are four atoms per FCC unit cell has several significant consequences:
-
Atomic Packing Factor (APF): The APF represents the fraction of space within a unit cell that is occupied by atoms. In an FCC structure, the APF is remarkably high (approximately 0.74), indicating a very efficient packing arrangement. This high APF contributes to the relatively high density observed in many FCC metals.
-
Coordination Number: The coordination number refers to the number of nearest neighbor atoms surrounding a given atom. In an FCC structure, each atom is surrounded by twelve nearest neighbors, leading to strong bonding and influencing various material properties.
-
Material Properties: The FCC structure's efficient packing and high coordination number contribute to several important material properties, including:
- High ductility and malleability: The ability to deform plastically without fracturing.
- High electrical and thermal conductivity: Due to the close proximity of atoms facilitating electron movement.
- Relatively high density: A direct result of the efficient packing arrangement.
Beyond the Basics: Exploring Different Crystal Structures
While the FCC structure is common, it’s important to remember that it’s just one of several possible crystal structures. Understanding the atom count within other structures requires a similar analysis of atom positions and sharing between unit cells.
-
Body-Centered Cubic (BCC): In a BCC unit cell, atoms are located at each corner and one atom in the center of the cube. Each corner atom is shared by eight unit cells (contributing 1 atom), and the center atom belongs entirely to the unit cell (contributing 1 atom). Therefore, a BCC unit cell contains a total of two atoms.
-
Simple Cubic (SC): This structure has atoms only at the corners of the cube. Each corner atom is shared by eight unit cells, resulting in a total of one atom per unit cell.
The differences in atom arrangements and the resulting atom counts per unit cell directly impact the physical and chemical properties of materials.
Practical Applications and Examples of FCC Materials
Many common metals and alloys adopt the FCC structure. Understanding the characteristics of this structure is crucial for numerous applications:
-
Aluminum: Widely used in aerospace, automotive, and packaging industries due to its lightness, strength, and corrosion resistance.
-
Copper: Essential in electrical wiring and plumbing due to its excellent electrical and thermal conductivity.
-
Gold: Highly valued for its chemical inertness, malleability, and aesthetic appeal, used in jewelry and electronics.
-
Nickel: Used in various alloys, including stainless steel, due to its strength, corrosion resistance, and magnetic properties.
-
Silver: Similar to copper and gold, valued for its conductivity and used in electronics and jewelry.
The properties of these materials are directly linked to their FCC crystal structure and the resulting four atoms per unit cell.
Advanced Considerations: Defects and Imperfections
Real-world crystals are not perfect; they contain various defects and imperfections. These defects can significantly influence the material's properties. While our calculations assume a perfect, ideal crystal, it's important to acknowledge that real materials deviate from this ideal. Point defects, line defects (dislocations), and planar defects all contribute to the complexity of real-world crystal structures. These imperfections affect the number of atoms in a given volume and also influence the material properties. Accounting for these defects requires sophisticated techniques beyond the scope of this basic introduction.
Conclusion: The Significance of Atomic Arrangement in Materials Science
The seemingly simple question of how many atoms are in an FCC unit cell leads us to a deep understanding of crystallography and its significance in materials science. The precise arrangement of atoms within the unit cell – in this case, resulting in four atoms per FCC unit cell – directly impacts the material's density, packing efficiency, coordination number, and ultimately, its macroscopic properties. This knowledge is fundamental to material selection, design, and processing in various engineering and scientific applications. Understanding the FCC structure and similar crystal lattices is essential for anyone working with materials, offering insights into their behavior and potential applications. The exploration into BCC and SC structures further broadens our understanding of how atomic arrangement dictates material properties. Finally, acknowledging the complexities introduced by real-world imperfections emphasizes the need for advanced techniques and considerations when dealing with real materials.
Latest Posts
Latest Posts
-
What Is 6 25 As A Fraction
Mar 28, 2025
-
Which Of The Following Compounds Is Ionic
Mar 28, 2025
-
Which Characteristic Is Common To All Chordates
Mar 28, 2025
-
Give The Major Product For The Following Reaction
Mar 28, 2025
-
Is India In The Northern Or Southern Hemisphere
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In A Fcc Unit Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
