How Is Temperature Related To The Motions Of Molecules
News Leon
Mar 31, 2025 · 6 min read
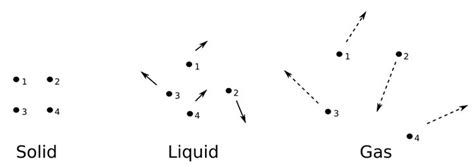
Table of Contents
How is Temperature Related to the Motions of Molecules?
Temperature, a fundamental concept in physics and everyday life, is intrinsically linked to the microscopic world of molecules and their ceaseless motion. Understanding this relationship is key to comprehending various phenomena, from the boiling of water to the behavior of gases in engines. This article delves deep into the connection between temperature and molecular motion, exploring the kinetic theory of gases, the different phases of matter, and the implications of this relationship for various scientific fields.
The Kinetic Theory of Gases: A Foundation for Understanding
The kinetic theory of gases provides a robust framework for explaining the relationship between temperature and molecular motion. This theory rests on several key postulates:
-
Gases are composed of tiny particles (atoms or molecules) that are in constant, random motion. These particles are constantly colliding with each other and with the walls of their container.
-
The volume occupied by the gas particles themselves is negligible compared to the volume of the container. This means that the particles are considered to be point masses, significantly simplifying calculations.
-
The forces of attraction or repulsion between gas particles are negligible. This assumption is most accurate for ideal gases, although real gases deviate from ideal behavior, especially at high pressures and low temperatures.
-
Collisions between gas particles and between particles and the container walls are perfectly elastic. This means that kinetic energy is conserved during collisions; no energy is lost as heat.
-
The average kinetic energy of the gas particles is directly proportional to the absolute temperature of the gas. This is the crucial link between temperature and molecular motion. The higher the temperature, the faster the particles move, and the greater their average kinetic energy.
Absolute Temperature and Kinetic Energy: A Direct Relationship
The relationship between average kinetic energy and absolute temperature (measured in Kelvin) is expressed mathematically as:
KE<sub>avg</sub> = (3/2)kT
Where:
- KE<sub>avg</sub> is the average kinetic energy of the gas particles
- k is the Boltzmann constant (a fundamental physical constant)
- T is the absolute temperature in Kelvin
This equation underscores the direct proportionality: as absolute temperature increases, so does the average kinetic energy of the gas particles. This increase in kinetic energy manifests as faster, more energetic molecular motion.
Temperature and the States of Matter: Solids, Liquids, and Gases
The relationship between temperature and molecular motion significantly impacts the physical state of matter. Different states exhibit distinct characteristics regarding molecular movement and interactions:
Solids: Restricted Motion
In solids, molecules are tightly packed together and experience strong intermolecular forces. Their motion is largely restricted to vibrations around fixed equilibrium positions. While molecules do vibrate, their movements are relatively small and constrained by the rigid structure of the solid. As temperature increases, the amplitude of these vibrations increases, leading to expansion of the solid.
Liquids: Increased Freedom of Movement
Liquids exhibit a greater degree of molecular freedom compared to solids. Molecules are still relatively close together, but they can move and slide past each other. This allows for fluidity and the ability to conform to the shape of their container. Higher temperatures increase the kinetic energy of the molecules, leading to greater mobility and decreased viscosity.
Gases: Independent and Random Motion
In gases, molecules are widely dispersed, with weak intermolecular forces. They move independently and randomly, undergoing frequent collisions with each other and the container walls. The average kinetic energy, and therefore the speed of the molecules, is directly proportional to the absolute temperature. Higher temperatures lead to faster molecular speeds and higher pressure due to increased collisions with the container walls.
Beyond Ideal Gases: Real Gases and Intermolecular Forces
The kinetic theory of gases, as discussed above, describes ideal gases. Real gases, however, deviate from ideal behavior, particularly at high pressures and low temperatures. This deviation stems from the presence of intermolecular forces—attractive forces between molecules—which the ideal gas model neglects.
At low temperatures, intermolecular forces become more significant, leading to a reduction in the average kinetic energy and a decrease in the speed of the molecules. This can result in condensation, where the gas transitions into a liquid state. High pressures also bring molecules closer together, increasing the impact of intermolecular forces and leading to deviations from ideal gas behavior.
Temperature and Phase Transitions: A Dynamic Equilibrium
Phase transitions, such as melting, boiling, and sublimation, represent dramatic changes in the state of matter driven by temperature changes and the corresponding alterations in molecular motion. These transitions occur at specific temperatures and pressures, reflecting the balance between kinetic energy and intermolecular forces.
-
Melting: As the temperature of a solid increases, the vibrational energy of its molecules overcomes the intermolecular forces holding them in fixed positions. This leads to a transition from a solid to a liquid, where molecules have greater freedom of movement.
-
Boiling: Further increases in temperature result in increased molecular kinetic energy, eventually leading to the boiling point. At this point, the kinetic energy is sufficient to overcome the intermolecular forces completely, and the liquid transitions to a gas, with molecules exhibiting independent, random motion.
-
Sublimation: Under certain conditions, a solid can directly transition to a gas without passing through the liquid phase. This process, known as sublimation, occurs when the vapor pressure of the solid exceeds the atmospheric pressure, allowing molecules to escape directly into the gaseous phase.
Applications and Implications: From Everyday Life to Advanced Research
The relationship between temperature and molecular motion has vast implications across numerous scientific disciplines and everyday applications:
-
Thermodynamics: Temperature is a central parameter in thermodynamics, governing the direction and extent of heat transfer and work done in various processes. Understanding molecular motion provides a microscopic basis for macroscopic thermodynamic principles.
-
Chemistry: Chemical reactions depend heavily on the kinetic energy of reacting molecules. Temperature influences the rate of reactions by affecting the frequency and energy of collisions between molecules.
-
Material Science: The properties of materials, such as strength, elasticity, and conductivity, are significantly affected by temperature and the corresponding changes in molecular arrangement and motion.
-
Meteorology: Understanding the movement of air molecules is crucial for weather forecasting and climate modeling. Temperature gradients drive atmospheric circulation patterns and influence weather systems.
-
Biology: Biological processes are highly sensitive to temperature. Enzyme activity, cellular metabolism, and overall organism function are all influenced by temperature-dependent changes in molecular motion.
-
Engineering: The design of engines, refrigerators, and other thermal systems heavily relies on understanding the relationship between temperature and molecular motion to optimize efficiency and performance.
Conclusion: A Fundamental Connection with Far-Reaching Consequences
The relationship between temperature and molecular motion is a fundamental concept in physics and chemistry, with broad-reaching consequences in numerous scientific fields and everyday applications. Understanding this relationship allows us to comprehend the behavior of matter in different states, the dynamics of phase transitions, and the intricacies of various physical and chemical processes. From the seemingly simple act of boiling water to the complex mechanisms of cellular processes, the constant dance of molecules driven by temperature plays a crucial role in shaping the world around us. Further research and exploration continue to reveal new facets of this fundamental connection, further expanding our understanding of the universe at both the macroscopic and microscopic levels.
Latest Posts
Latest Posts
-
What Is The Charge On A Sulfide Ion
Apr 01, 2025
-
What Is Not A Function Of The Lymphatic System
Apr 01, 2025
-
Who Has Hemophilia In The Pedigree That Is Shown
Apr 01, 2025
-
A Chord That Passes Through The Center Of A Circle
Apr 01, 2025
-
Which Of The Following Is A Fixed Cost
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Is Temperature Related To The Motions Of Molecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
