How Does Atp Release Energy That's Stored Within The Molecule
News Leon
Apr 06, 2025 · 5 min read
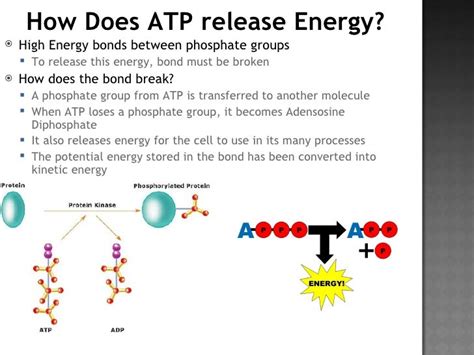
Table of Contents
How Does ATP Release Energy Stored Within the Molecule?
Adenosine triphosphate, or ATP, is the primary energy currency of all living cells. Understanding how ATP releases its stored energy is fundamental to comprehending cellular processes, from muscle contraction to nerve impulse transmission. This process isn't a simple "explosion" of energy, but a carefully controlled chemical reaction that allows cells to harness energy precisely when and where it's needed. This article will delve into the intricate mechanism of ATP energy release, exploring the molecular interactions and the crucial role of enzymes.
The Structure of ATP: A Molecular Energy Storage Unit
Before exploring energy release, let's examine ATP's structure. ATP is a nucleotide composed of three main components:
- Adenine: A nitrogenous base, a crucial part of DNA and RNA.
- Ribose: A five-carbon sugar, forming the backbone of the molecule.
- Triphosphate: A chain of three phosphate groups linked together. It's the triphosphate tail that holds the key to ATP's energy storage capacity.
The phosphate groups are negatively charged and are closely packed together. This proximity creates significant electrostatic repulsion. This repulsion is a major source of potential energy within the ATP molecule. Think of it like a tightly compressed spring – the more compressed, the more potential energy it stores. Breaking this "spring" – the phosphate bonds – releases this stored energy.
Hydrolysis of ATP: The Key to Energy Release
The primary mechanism by which ATP releases its energy is through a process called hydrolysis. Hydrolysis is a chemical reaction where water is used to break a chemical bond. In the case of ATP, the hydrolysis involves breaking a high-energy phosphate bond, specifically the bond between the second and third phosphate groups. This reaction produces:
- Adenosine diphosphate (ADP): ATP minus one phosphate group.
- Inorganic phosphate (Pi): The cleaved phosphate group, often written as Pi.
- Energy: Released in the form of free energy, available to drive cellular processes.
The reaction can be represented as follows:
ATP + H₂O → ADP + Pi + Energy
The Role of Enzymes: ATPases
The hydrolysis of ATP doesn't spontaneously occur at a significant rate under cellular conditions. The reaction requires the assistance of enzymes, specifically a class of enzymes called ATPases. ATPases catalyze the hydrolysis of ATP, significantly accelerating the reaction rate and making it efficient enough for cellular needs.
Different ATPases have different functions and mechanisms, but they all share the common goal of facilitating ATP hydrolysis and harnessing the released energy. For instance:
- Myosin ATPase in muscle cells utilizes the energy from ATP hydrolysis to power muscle contraction.
- Sodium-potassium pump (Na+/K+-ATPase) uses ATP hydrolysis to maintain the electrochemical gradient across cell membranes, crucial for nerve impulse transmission and other processes.
- DNA polymerase utilizes the energy from ATP hydrolysis (or other nucleoside triphosphates like GTP) during DNA replication to link nucleotides together.
Why is the Phosphate Bond High-Energy?
The energy released during ATP hydrolysis is significant due to several factors:
- Electrostatic repulsion: As mentioned earlier, the negative charges of the phosphate groups repel each other strongly. Removing a phosphate group relieves this repulsion, releasing a considerable amount of energy.
- Resonance stabilization: The products of ATP hydrolysis, ADP and Pi, are more resonance-stabilized than ATP. Resonance stabilization refers to the delocalization of electrons, increasing the molecule's stability and lowering its energy. The increased stability of the products contributes to the overall energy release.
- Solvation: ADP and Pi are more readily hydrated (surrounded by water molecules) than ATP. This increased hydration further stabilizes the products, contributing to the exergonic nature of the reaction.
Beyond Hydrolysis: Other Ways ATP Releases Energy
While hydrolysis is the primary method, ATP can also release energy through other mechanisms, although these are less common:
- Phosphorylation: ATP can directly transfer a phosphate group to another molecule, a process called phosphorylation. This process activates or modifies the target molecule, often an enzyme, changing its activity. The transfer of the phosphate group is coupled with an energy transfer, driving the cellular process.
- Pyrophosphate cleavage: Less common than hydrolysis, the cleavage of the bond between the alpha and beta phosphates (releasing pyrophosphate, PPi) can also release energy.
The ATP Cycle: A Continuous Energy Renewal
The energy released during ATP hydrolysis is crucial, but the supply of ATP itself needs to be constantly replenished. This is achieved through the ATP cycle, a continuous process involving ATP synthesis and hydrolysis.
ATP synthesis occurs primarily through:
- Cellular respiration: The process of breaking down glucose and other fuels to generate ATP. This occurs in the mitochondria through oxidative phosphorylation.
- Photosynthesis: In plants and some other organisms, photosynthesis uses sunlight to generate ATP.
The ATP cycle ensures a steady supply of energy currency for the cell's numerous energy-demanding processes. The constant interplay between ATP synthesis and hydrolysis maintains cellular energy homeostasis.
ATP and Cellular Work: Diverse Applications
ATP's energy release fuels a vast array of cellular processes, including:
- Muscle contraction: ATP powers the interaction between actin and myosin filaments, leading to muscle shortening and movement.
- Active transport: ATP drives the movement of ions and molecules across cell membranes against their concentration gradients, maintaining cellular homeostasis.
- Protein synthesis: ATP is required for the formation of peptide bonds during protein synthesis.
- Signal transduction: ATP plays a role in various signaling pathways, transferring energy to activate downstream processes.
- Nerve impulse transmission: ATP is crucial for maintaining the electrochemical gradient across nerve cell membranes and driving the transmission of nerve impulses.
- DNA replication and repair: ATP (and other nucleoside triphosphates) fuels the enzymatic processes involved in DNA replication and repair.
Conclusion: ATP – The Cell's Powerhouse
The release of energy from ATP is a finely tuned process, essential for the survival and function of all living cells. Hydrolysis, catalyzed by ATPases, is the primary mechanism, driven by electrostatic repulsion, resonance stabilization, and solvation effects. This energy is then harnessed to drive a myriad of essential cellular processes. Understanding this process is paramount to understanding how life itself functions at a molecular level. Further research continues to unravel the intricacies of ATP metabolism and its vital role in maintaining the dynamic equilibrium of cellular life. The continuous cycling of ATP synthesis and hydrolysis ensures a constant supply of energy, fueling the intricate machinery of the cell. This elegant molecular mechanism is a testament to the remarkable efficiency and precision of biological systems.
Latest Posts
Latest Posts
-
The Oxygen Released During Photosynthesis Comes From
Apr 07, 2025
-
Is Cellular Respiration A Chemical Change
Apr 07, 2025
-
What Is The Oxidation State Of Chlorine In Hclo2
Apr 07, 2025
-
What Ions Are Produced From Acids And From Bases
Apr 07, 2025
-
Is Baking Soda A Mixture Or Compound
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about How Does Atp Release Energy That's Stored Within The Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
