Is Baking Soda A Mixture Or Compound
News Leon
Apr 07, 2025 · 5 min read
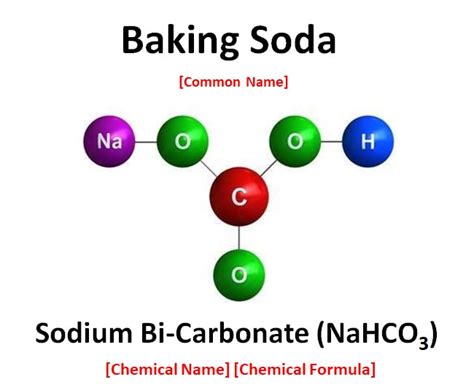
Table of Contents
Is Baking Soda a Mixture or a Compound? A Deep Dive into Chemical Composition
Baking soda, a ubiquitous ingredient in countless kitchens worldwide, is more than just a leavening agent. Understanding its chemical nature – whether it's a mixture or a compound – opens the door to appreciating its versatile applications and unique properties. This comprehensive exploration will delve into the intricacies of baking soda's composition, clarifying its classification and explaining the key differences between mixtures and compounds. We’ll also investigate the implications of its chemical structure for its various uses, from baking to cleaning and beyond.
Understanding the Difference: Mixtures vs. Compounds
Before we can definitively classify baking soda, let's establish a clear understanding of the fundamental differences between mixtures and compounds.
Mixtures: A Blend of Substances
A mixture is a physical combination of two or more substances that are not chemically bonded. The individual components retain their unique chemical properties and can be separated through physical means, such as filtration, distillation, or evaporation. Examples of mixtures include saltwater (salt and water), air (a mixture of gases), and sand (a mixture of different minerals). Crucially, the composition of a mixture can vary. A cup of saltwater can have a different concentration of salt than another cup.
Compounds: Chemically Bonded Substances
A compound, conversely, is a chemical combination of two or more elements that are chemically bonded in fixed proportions. These bonds create a new substance with entirely different properties from its constituent elements. For example, water (H₂O) is a compound formed from the elements hydrogen and oxygen. It has different properties than hydrogen or oxygen alone. The composition of a compound is always consistent; water is always H₂O, never a variable mixture of hydrogen and oxygen.
The Chemical Identity of Baking Soda: Sodium Bicarbonate
Baking soda's chemical name is sodium bicarbonate, with the formula NaHCO₃. This formula reveals its composition: one sodium atom (Na), one hydrogen atom (H), one carbon atom (C), and three oxygen atoms (O). These elements are chemically bonded together, forming a distinct chemical entity.
Why baking soda isn't a mixture:
The key point here is the chemical bonding. The atoms in sodium bicarbonate aren't simply mixed together; they are linked through strong chemical bonds. You cannot physically separate sodium, hydrogen, carbon, and oxygen from sodium bicarbonate without breaking these chemical bonds through a chemical reaction. This is the defining characteristic of a compound. Methods like filtering or evaporation would not work; they are only effective in separating components of a mixture.
Evidence supporting the compound classification:
Several pieces of evidence reinforce baking soda's classification as a compound:
- Fixed Composition: Sodium bicarbonate always has the same chemical formula, NaHCO₃. No matter the source or method of production, the ratio of sodium, hydrogen, carbon, and oxygen remains constant.
- Unique Properties: Baking soda possesses unique properties distinct from its constituent elements. Sodium is a highly reactive metal, while hydrogen and oxygen are gases. Baking soda is a white crystalline powder, a leavening agent, and a mild base, properties none of its constituent elements possess individually.
- Chemical Reactions: Baking soda undergoes characteristic chemical reactions, such as reacting with acids to produce carbon dioxide gas (a key component of its leavening action in baking). These reactions are evidence of its chemical nature.
Baking Soda's Applications: A Consequence of its Chemical Nature
The versatility of baking soda stems directly from its chemical structure and properties as a compound:
Baking: The Leavening Power
Baking soda’s most common use is as a leavening agent in baking. When combined with an acidic ingredient (like vinegar, buttermilk, or lemon juice), it undergoes a chemical reaction that produces carbon dioxide gas. This gas expands within the batter or dough, creating air pockets that make baked goods light and fluffy. The specific chemical reaction is an acid-base neutralization reaction.
Cleaning: A Mild Abrasive and Deodorizer
Baking soda's mild abrasive properties make it an effective cleaning agent. Its fine crystals can scrub away dirt and grime without scratching surfaces. Furthermore, its ability to neutralize acids makes it useful for cleaning acidic spills. Its alkaline nature can also neutralize odors, making it effective as a deodorizer.
Health and Personal Care: Antacid and Toothpaste Ingredient
Baking soda’s mild alkaline nature makes it a useful antacid to relieve heartburn and indigestion. It neutralizes stomach acid, providing temporary relief. Its gentle abrasive properties also contribute to its use in some toothpastes, helping to remove surface stains from teeth. However, it's crucial to remember that it's not a substitute for professional dental care.
Other Uses: Fire Extinguishers and Water Treatment
Baking soda's ability to react with acids and extinguish flames has led to its use in some fire extinguishers. It also plays a role in water treatment, helping to adjust the pH of water.
Common Misconceptions about Baking Soda
Despite the straightforward chemical nature of baking soda, some common misconceptions persist:
-
Confusion with Baking Powder: Baking powder is not the same as baking soda. Baking powder contains both baking soda and an acidic component, whereas baking soda alone requires a separate acidic ingredient to react and produce carbon dioxide.
-
Overestimating its Cleaning Power: While baking soda is a useful cleaning agent, it's not a miracle cleaner. Stubborn stains or heavily soiled areas might require stronger cleaning agents.
Conclusion: Baking Soda – A Compound with Multifaceted Uses
In conclusion, baking soda (sodium bicarbonate) is unequivocally a compound, not a mixture. Its atoms are chemically bonded in a fixed ratio, forming a unique substance with distinctive properties. This chemical nature underpins its versatile applications in baking, cleaning, health, and other industries. Understanding the difference between mixtures and compounds, and appreciating the unique chemical composition of baking soda, allows for a deeper understanding of its functionality and its crucial role in many aspects of daily life. From the fluffy texture of your cakes to the sparkle of your clean countertops, baking soda’s powerful effects are a direct result of its fascinating chemical identity as a compound.
Latest Posts
Latest Posts
-
Diffusion And Facilitated Diffusion Are Both
Apr 11, 2025
-
Which One Of The Following Is A True Statement
Apr 11, 2025
-
Geometric Mean Of 5 And 20
Apr 11, 2025
-
Is Methane A Compound Or Element
Apr 11, 2025
-
An Electron Has An Initial Velocity
Apr 11, 2025
Related Post
Thank you for visiting our website which covers about Is Baking Soda A Mixture Or Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.