What Ions Are Produced From Acids And From Bases
News Leon
Apr 07, 2025 · 6 min read
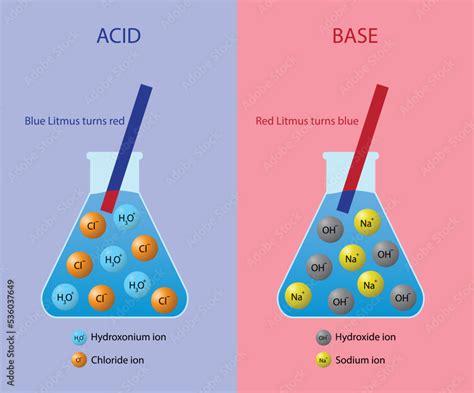
Table of Contents
What Ions are Produced from Acids and from Bases?
Understanding the ions produced by acids and bases is fundamental to grasping the concepts of acid-base chemistry. This knowledge underpins numerous applications, from everyday phenomena like digestion to sophisticated industrial processes. This comprehensive guide will delve into the ionic nature of acids and bases, exploring the specific ions they generate in solution and the implications of these dissociations.
Defining Acids and Bases: The Arrhenius Perspective
Before examining the ions themselves, let's establish a clear definition of acids and bases. The Arrhenius theory, a cornerstone of acid-base chemistry, provides a simple yet effective framework. According to Arrhenius:
- An acid is a substance that produces hydrogen ions (H⁺) when dissolved in water.
- A base is a substance that produces hydroxide ions (OH⁻) when dissolved in water.
This definition effectively explains the properties of many common acids and bases. For example, hydrochloric acid (HCl) dissolves in water to yield H⁺ and Cl⁻ ions, while sodium hydroxide (NaOH) dissociates to form Na⁺ and OH⁻ ions. However, the Arrhenius definition has limitations; it doesn't encompass all substances exhibiting acidic or basic behavior. We'll explore broader definitions later.
Strong vs. Weak Acids and Bases: Degree of Dissociation
The extent to which an acid or base dissociates into ions in water determines its strength.
-
Strong acids and bases completely dissociate into their constituent ions in aqueous solution. This means virtually every molecule of the acid or base breaks apart into ions. Examples of strong acids include HCl (hydrochloric acid), HNO₃ (nitric acid), and H₂SO₄ (sulfuric acid). Examples of strong bases include NaOH (sodium hydroxide), KOH (potassium hydroxide), and Ca(OH)₂ (calcium hydroxide).
-
Weak acids and bases only partially dissociate in water. A significant portion of the molecules remain undissociated, existing in equilibrium with the ions they produce. This equilibrium is crucial, as it determines the acidity or basicity of the solution. Examples of weak acids include acetic acid (CH₃COOH) and carbonic acid (H₂CO₃). Examples of weak bases include ammonia (NH₃) and methylamine (CH₃NH₂).
The difference in behavior between strong and weak acids/bases significantly impacts the properties of their solutions, such as pH and conductivity. Strong acids and bases are better conductors of electricity due to the high concentration of ions.
Ions Produced by Acids: Beyond H⁺
While the Arrhenius definition emphasizes the production of H⁺ ions, it's essential to remember that the hydrogen ion, in reality, doesn't exist independently in aqueous solution. Instead, it readily reacts with a water molecule to form a hydronium ion (H₃O⁺). This reaction is crucial because it reflects the true nature of proton transfer in aqueous solutions. Therefore, when discussing the ions produced by acids, it's more accurate to consider the hydronium ion rather than the bare proton.
The other ion produced depends entirely on the specific acid. For example:
- HCl (hydrochloric acid): Produces H₃O⁺ and Cl⁻ ions.
- HNO₃ (nitric acid): Produces H₃O⁺ and NO₃⁻ ions.
- H₂SO₄ (sulfuric acid): Produces H₃O⁺ and HSO₄⁻ ions (in the first dissociation step). The HSO₄⁻ ion can further dissociate to produce another H₃O⁺ and SO₄²⁻.
- CH₃COOH (acetic acid): Produces H₃O⁺ and CH₃COO⁻ ions (in a small equilibrium).
The anion (negatively charged ion) produced by the acid is the conjugate base of that acid. The conjugate base is what remains after the acid has donated a proton.
Ions Produced by Bases: Beyond OH⁻
Similarly, while the Arrhenius definition focuses on OH⁻ production, understanding the broader context is crucial. Many substances behave as bases without directly producing hydroxide ions.
-
Group 1 and 2 hydroxides: These strong bases directly dissociate to produce hydroxide ions (OH⁻) and a metal cation (e.g., Na⁺ from NaOH, Ca²⁺ from Ca(OH)₂).
-
Ammonia (NH₃) and other amines: These act as weak bases through a process called proton acceptance. Ammonia reacts with water to form ammonium (NH₄⁺) and hydroxide (OH⁻) ions:
NH₃ + H₂O ⇌ NH₄⁺ + OH⁻
This equilibrium demonstrates that ammonia doesn't directly produce OH⁻ but rather generates it through its reaction with water. Amines, derivatives of ammonia, behave similarly.
-
Other bases: Many substances can act as bases without directly producing OH⁻. These bases accept protons from water or other acids, leading to an increase in the concentration of OH⁻ ions. This is particularly relevant when considering the Brønsted-Lowry and Lewis acid-base theories.
Beyond Arrhenius: Broader Definitions of Acids and Bases
The Arrhenius definition, while helpful, has limitations. Two more comprehensive definitions offer a broader perspective:
-
Brønsted-Lowry theory: Defines acids as proton donors and bases as proton acceptors. This definition extends acid-base reactions beyond aqueous solutions. A Brønsted-Lowry acid donates a proton (H⁺) to a Brønsted-Lowry base. For example, in the reaction between HCl and NH₃, HCl acts as the acid (proton donor) and NH₃ acts as the base (proton acceptor), forming NH₄⁺ and Cl⁻. Note that this definition doesn't require the presence of water.
-
Lewis theory: The most general definition, it defines acids as electron-pair acceptors and bases as electron-pair donors. This theory encompasses a vast range of reactions that don't involve protons. A Lewis acid accepts a pair of electrons from a Lewis base to form a coordinate covalent bond. For example, BF₃ (a Lewis acid) reacts with NH₃ (a Lewis base) where the nitrogen atom in NH₃ donates a lone pair of electrons to the boron atom in BF₃.
These expanded definitions show that many substances can act as acids or bases, not just those producing H⁺ or OH⁻ in water.
The Importance of Understanding Acid and Base Dissociation
Understanding the ions produced by acids and bases is crucial for several reasons:
- Predicting reaction outcomes: Knowing the ions involved allows us to predict the products of acid-base reactions and their properties.
- Calculating pH: The concentration of H₃O⁺ ions (or H⁺) directly determines the pH of a solution, a measure of its acidity or basicity.
- Understanding biological processes: Many biological processes rely on acid-base reactions, such as enzyme function and blood pH regulation.
- Industrial applications: Acid-base reactions are central to many industrial processes, including chemical synthesis, wastewater treatment, and food processing.
- Environmental monitoring: Monitoring the acidity or basicity of water bodies is crucial for environmental protection.
Conclusion
The ions produced by acids and bases are central to understanding acid-base chemistry. While the Arrhenius definition offers a simple introduction, the Brønsted-Lowry and Lewis theories provide a more comprehensive framework. Understanding the dissociation of strong and weak acids and bases, the formation of hydronium ions, and the diverse ways bases can function is vital for comprehending a wide array of chemical phenomena and their applications in various fields. This knowledge forms the foundation for further exploration into the fascinating world of acid-base reactions and their significance in our world.
Latest Posts
Latest Posts
-
All Of The Following Are Forms Of Nonverbal Communication Except
Apr 11, 2025
-
Why Is Coal Not Classified As A Mineral
Apr 11, 2025
-
Part Of The Brain That Controls Heartbeat
Apr 11, 2025
-
The Charge To Mass Ratio Of An Electron Is
Apr 11, 2025
-
The Most Abundant Element In The Air
Apr 11, 2025
Related Post
Thank you for visiting our website which covers about What Ions Are Produced From Acids And From Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.