Give The Iupac Name For The Following Compound. 2xsafari
News Leon
Mar 30, 2025 · 5 min read
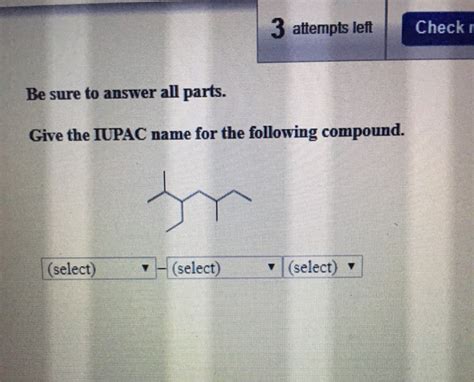
Table of Contents
Giving IUPAC Names: A Deep Dive into Organic Nomenclature with 2xSafari Example
The world of organic chemistry can seem daunting, a vast landscape of carbon chains, functional groups, and complex structures. However, navigating this landscape becomes significantly easier with a firm grasp of IUPAC nomenclature – the international standard for naming organic compounds. This systematic approach allows chemists worldwide to unambiguously identify and communicate about even the most complex molecules. Let's explore the principles of IUPAC nomenclature, using a hypothetical example "2xSafari" (which we will interpret as a specific chemical structure) to illustrate the process. We will dissect the naming conventions step-by-step, ensuring a clear understanding of how to assign IUPAC names.
Understanding the Fundamentals of IUPAC Nomenclature
Before tackling our example, let's review the essential building blocks of IUPAC naming:
-
Parent Chain: This is the longest continuous carbon chain within the molecule. It forms the base name of the compound.
-
Substituents: These are atoms or groups of atoms attached to the parent chain. They are named individually and their positions on the chain are indicated by numbers.
-
Functional Groups: These are specific groups of atoms within a molecule that confer characteristic chemical properties. They are given priority in naming and often dictate the suffix of the IUPAC name.
-
Numbering: The carbon atoms in the parent chain are numbered to indicate the position of substituents. Numbering should be done in a way that gives the substituents the lowest possible numbers.
-
Alphabetical Order: Substituents are listed alphabetically before the parent chain name, ignoring prefixes like di-, tri-, etc. (except for iso, sec, and tert).
Deciphering "2xSafari": A Hypothetical Example
Let's assume "2xSafari" represents a molecule with two identical substituents (indicated by "2x") and a parent chain suggested by "Safari" (which we will interpret for the sake of this example). We need to make assumptions about the specific structure based on this naming, as it's not standard chemical nomenclature.
Let's propose a possible structure:
Assume "Safari" represents a 6-carbon chain (hexane) and the substituent represented by "x" is a methyl group (-CH3). "2x" indicates there are two methyl groups. Therefore, our hypothetical "2xSafari" molecule could be 2,3-dimethylhexane.
This interpretation allows us to demonstrate the step-by-step application of IUPAC rules.
Step-by-Step IUPAC Naming of 2,3-Dimethylhexane
-
Identify the Parent Chain: The longest continuous carbon chain contains six carbon atoms; this is a hexane.
-
Identify the Substituents: Two methyl groups (-CH3) are attached to the parent chain.
-
Number the Carbon Atoms: We number the carbon atoms in the hexane chain to give the methyl groups the lowest possible numbers. In this case, numbering from left to right yields 2,3-dimethyl.
-
Arrange the Substituents Alphabetically: There's only one type of substituent here, so no alphabetical ordering is necessary.
-
Write the IUPAC Name: Combining the substituent positions and names with the parent chain name, we arrive at the IUPAC name: 2,3-dimethylhexane.
Expanding on the Principles with More Complex Examples
Now let's move beyond our hypothetical "2xSafari" and explore more intricate examples to further solidify our understanding of IUPAC nomenclature.
Example 1: 3-Ethyl-2,4-dimethylheptane
-
Parent Chain: The longest continuous carbon chain contains seven carbon atoms (heptane).
-
Substituents: One ethyl group (-CH2CH3) and two methyl groups (-CH3) are present.
-
Numbering: Numbering the heptane chain provides the lowest possible numbers for the substituents: 2, 3, and 4.
-
Alphabetical Order: Ethyl comes before methyl alphabetically, so ethyl is mentioned first.
-
IUPAC Name: 3-Ethyl-2,4-dimethylheptane
Example 2: 4-Bromo-2-chloro-3-methylpentane
-
Parent Chain: The longest continuous chain has five carbons (pentane).
-
Substituents: One bromo group (-Br), one chloro group (-Cl), and one methyl group (-CH3) are attached.
-
Numbering: Numbering assigns the lowest possible numbers (2, 3, and 4).
-
Alphabetical Order: Bromo is placed before chloro and methyl in the name.
-
IUPAC Name: 4-Bromo-2-chloro-3-methylpentane
Example 3: A Compound with Multiple Functional Groups
When multiple functional groups are present, a priority order is used, influencing both the suffix and prefix:
Consider a molecule with a carboxylic acid (-COOH) and an alcohol (-OH) functional group. Carboxylic acids have higher priority than alcohols. The parent chain is named based on the longest chain including the carboxylic acid carbon. The alcohol becomes a hydroxy substituent. For example, if the molecule is a 4-carbon chain with a carboxylic acid at position 1 and an alcohol at position 3:
The IUPAC name would be 3-hydroxybutanoic acid.
Incorporating Stereoisomers into IUPAC Nomenclature
For molecules with chiral centers, the IUPAC name incorporates stereochemical descriptors such as R, S, E, and Z. This adds another layer of complexity but ensures complete description of the molecule's three-dimensional structure.
Consider 2-bromobutane. This molecule has a chiral center at carbon 2. Depending on the arrangement of atoms around this center, it can exist as two enantiomers. Their IUPAC names would be (R)-2-bromobutane and (S)-2-bromobutane. The (R) and (S) descriptors indicate the absolute configuration of the chiral center using the Cahn-Ingold-Prelog priority rules.
For alkenes with double bonds, E/Z notation describes the relative configuration of substituents about the double bond.
Advanced Aspects of IUPAC Nomenclature
The examples above demonstrate the core principles. More complex molecules might necessitate the use of additional rules and nomenclature systems to handle branched substituents, cyclic structures, and complex functional group combinations.
Resources for Further Learning
Mastering IUPAC nomenclature requires practice and familiarity with the rules. Many online resources and textbooks offer detailed explanations and practice exercises. Working through numerous examples is crucial to developing proficiency.
Conclusion: The Power of Systematic Naming
The IUPAC naming system is far more than just a set of rules; it's the cornerstone of effective communication in organic chemistry. By systematically assigning names, chemists worldwide can confidently share information, collaborate on research, and advance our understanding of the intricate world of molecules. While initially challenging, mastering IUPAC nomenclature unlocks a deeper appreciation for the structure and properties of organic compounds. Remember, practice is key. With consistent effort, the seemingly complex system becomes manageable and rewarding. The ability to accurately name and interpret the names of organic compounds is an essential skill for any aspiring chemist.
Latest Posts
Latest Posts
-
Which Of The Following Organisms Can Fix Nitrogen
Apr 01, 2025
-
Which Of The Following Is Correct About Viruses
Apr 01, 2025
-
How To Find Density Of Air
Apr 01, 2025
-
Which Of The Following Are Disaccharides
Apr 01, 2025
-
Which Of The Following Statements About Carbon Are True
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Give The Iupac Name For The Following Compound. 2xsafari . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
