How To Find Density Of Air
News Leon
Apr 01, 2025 · 6 min read
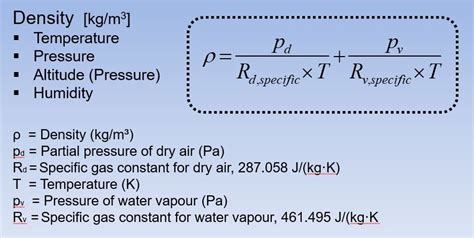
Table of Contents
How to Find the Density of Air: A Comprehensive Guide
Determining the density of air might seem like a niche topic, but it's crucial in various fields, from aerospace engineering and meteorology to industrial processes and even scuba diving. Understanding air density allows for accurate calculations involving buoyancy, pressure, and fluid dynamics. This comprehensive guide will delve into the methods for finding the air density, covering theoretical calculations, practical measurements, and the factors that influence this seemingly simple value.
Understanding Air Density
Before diving into the methods, let's establish a fundamental understanding. Air density (ρ), usually expressed in kilograms per cubic meter (kg/m³), represents the mass of air contained within a unit volume. Unlike liquids and solids, air density is highly variable, influenced by several environmental conditions.
Factors Affecting Air Density
Several key factors significantly impact air density:
- Temperature: As temperature rises, air molecules move faster and spread further apart, reducing density. Conversely, colder temperatures lead to denser air.
- Pressure: Higher atmospheric pressure forces air molecules closer together, resulting in increased density. Lower pressure leads to lower density.
- Humidity: Water vapor is lighter than dry air. Higher humidity means more water vapor, leading to slightly lower air density.
- Altitude: As altitude increases, atmospheric pressure decreases, resulting in a significant drop in air density. This is why high-altitude mountaineers experience breathing difficulties.
- Composition: Although the composition of air is relatively constant near the earth's surface, variations in the proportions of its constituent gases (nitrogen, oxygen, argon, carbon dioxide, etc.) can slightly influence its density.
Methods for Determining Air Density
There are two primary approaches to determining air density: calculation and measurement.
1. Calculating Air Density
This method is theoretical and relies on the ideal gas law and readily available environmental data. The formula is derived from the ideal gas law:
PV = nRT
Where:
- P is the atmospheric pressure (Pascals, Pa)
- V is the volume of air (cubic meters, m³)
- n is the number of moles of air
- R is the ideal gas constant (8.314 J/mol·K)
- T is the temperature in Kelvin (K)
To find density (ρ = m/V), we need to express the number of moles (n) in terms of mass (m) and molar mass (M):
n = m/M
Substituting this into the ideal gas law and rearranging to solve for density (ρ = m/V):
ρ = (P * M) / (R * T)
This formula is a powerful tool, but its accuracy depends on the accuracy of the input values (pressure, temperature, and molar mass).
Determining Molar Mass of Air
The molar mass of air (M) is not a constant value. It's approximately 28.97 g/mol, but this can vary slightly due to the aforementioned compositional changes. For most practical purposes, 28.97 g/mol is a sufficiently accurate approximation. However, for higher precision, a more detailed compositional analysis may be necessary.
Practical Application of the Calculation Method
Let's consider a practical example. Suppose you need to determine the air density at sea level on a day with the following conditions:
- Pressure (P): 101325 Pa (standard atmospheric pressure)
- Temperature (T): 20°C = 293.15 K
- Molar Mass (M): 28.97 g/mol = 0.02897 kg/mol
Plugging these values into the formula:
ρ = (101325 Pa * 0.02897 kg/mol) / (8.314 J/mol·K * 293.15 K)
ρ ≈ 1.204 kg/m³
This calculation provides a reasonable estimate of the air density under these specific conditions. Remember to always use consistent units.
2. Measuring Air Density
Directly measuring air density involves using specialized instruments. While less common than calculation, this method offers a more empirical approach, especially useful in situations where accurate environmental data isn't readily available or where the assumptions of the ideal gas law are not met.
Instruments for Measuring Air Density
Several instruments can directly or indirectly measure air density:
- Barometer and Thermometer: These are essential for obtaining the pressure and temperature required for calculating air density using the ideal gas law.
- Hygrometer: Measures humidity, enabling a more accurate calculation of air density by accounting for the presence of water vapor.
- Anemometer: While not directly measuring density, it measures wind speed, a factor that indirectly influences the dynamics of air and can be relevant in some applications.
- Specialized Air Density Meters: These instruments directly measure air density, often utilizing sophisticated sensors. However, these are generally expensive and not readily accessible for individual use.
Practical Application of the Measurement Method
The direct measurement method relies on the chosen instrument's instructions. However, generally, it involves calibrating the instrument, exposing it to the air sample, and reading the displayed density. Accuracy depends on instrument calibration and precision. A carefully calibrated air density meter can provide highly precise results.
Advanced Considerations
The methods discussed above utilize the ideal gas law, which assumes that air behaves as an ideal gas. While a good approximation for many situations, this assumption becomes less accurate under extreme conditions such as high pressure or low temperature.
Non-Ideal Gas Behavior
At high pressures or low temperatures, the interactions between air molecules become significant, deviating from the ideal gas law's assumptions. In such cases, more complex equations of state, such as the Van der Waals equation, are necessary to accurately determine air density. These equations account for the intermolecular forces and the finite volume of air molecules.
Altitude and Atmospheric Pressure
Air density significantly decreases with altitude. The calculation method using the ideal gas law requires accurate atmospheric pressure data for the specific altitude. Standard atmospheric models provide pressure profiles as a function of altitude.
Humidity Correction
The presence of water vapor significantly impacts air density. Accurately accounting for humidity requires precise measurements and adjustment to the ideal gas law calculation or using instruments capable of compensating for humidity.
Conclusion: Choosing the Right Method
The choice between calculating and measuring air density depends on several factors:
- Accuracy Requirements: For high accuracy, direct measurement with a calibrated instrument is preferable. For many applications, however, the calculation method provides sufficient accuracy.
- Resource Availability: Calculating air density requires readily available meteorological data. Direct measurement requires specialized equipment.
- Environmental Conditions: For extreme conditions where the ideal gas law is not a valid assumption, more advanced calculations or specialized equipment may be necessary.
By understanding the various factors that affect air density and mastering the methods for determining it, you'll gain valuable insights into a fundamental aspect of atmospheric science and a variety of applications across different fields. Remember to always check your units and ensure the accuracy of your input data for reliable results. Whether calculating or measuring, understanding the nuances and limitations of each method ensures accurate and reliable results in your specific application.
Latest Posts
Latest Posts
-
The Standard Unit For Measuring Volume Is
Apr 02, 2025
-
Materials Like Rubber That Resist The Flow Of E
Apr 02, 2025
-
What Are The Units Of Conductance
Apr 02, 2025
-
What Is Mega In Scientific Notation
Apr 02, 2025
-
Is Lead Sulphate Soluble In Water
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Find Density Of Air . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
