Give The Iupac Name For The Following Compound.
News Leon
Mar 15, 2025 · 8 min read
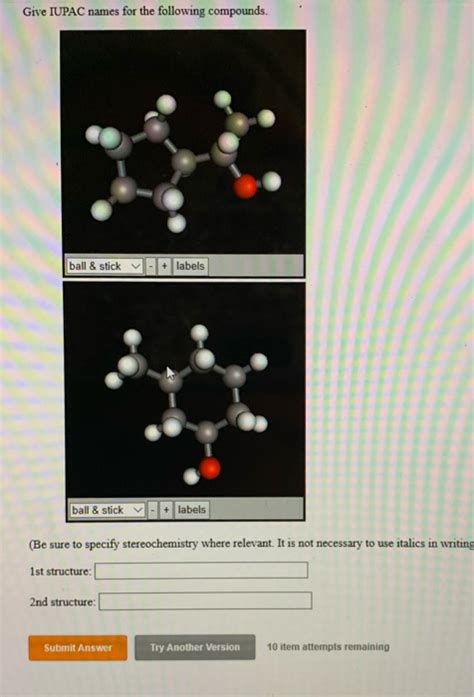
Table of Contents
Giving IUPAC Names to Organic Compounds: A Comprehensive Guide
Naming organic compounds might seem daunting at first, but with a systematic approach, it becomes a manageable and even enjoyable skill. The International Union of Pure and Applied Chemistry (IUPAC) provides a standardized nomenclature system to ensure unambiguous communication among chemists worldwide. This article delves into the intricacies of IUPAC nomenclature, providing a detailed guide to naming various organic compounds, complete with examples and explanations. We’ll cover alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, and more. Understanding this system is crucial for any student or professional in the field of chemistry.
Understanding the Basics of IUPAC Nomenclature
The IUPAC system is built upon a series of rules and priorities that dictate how we name organic molecules. The fundamental principle involves identifying the longest carbon chain (parent chain), identifying substituents attached to this chain, and numbering the carbon atoms in the chain to assign locations to these substituents. The name is then constructed by combining the names of the substituents with the name of the parent chain.
Identifying the Parent Chain
The parent chain is the longest continuous chain of carbon atoms in the molecule. This is crucial because the base name of the compound is derived from this chain. If there are multiple chains of equal length, the one with the most substituents is selected.
Example:
Consider the molecule: CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>
The longest carbon chain contains five carbon atoms, making it a pentane derivative.
Numbering the Carbon Chain
Once the parent chain is identified, the carbon atoms are numbered to assign locations to the substituents. Numbering begins from the end that gives the substituents the lowest possible numbers. If there are multiple substituents, the lowest number is assigned to the substituent with alphabetical priority (ignoring prefixes like di-, tri-, etc.).
Example:
In the molecule CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>, numbering from either end yields the same result: the methyl group is on carbon 2.
However, in a molecule like CH<sub>3</sub>CH(CH<sub>3</sub>)CH(C<sub>2</sub>H<sub>5</sub>)CH<sub>3</sub>, numbering from left to right gives a 2-methyl, 3-ethyl chain, while numbering from right to left yields a 3-methyl, 2-ethyl chain. The first option is preferred because 2 is less than 3.
Identifying and Naming Substituents
Substituents are atoms or groups of atoms that are attached to the parent chain. These substituents are named according to their structure. Common substituents include:
- Alkyl groups: These are derived from alkanes by removing a hydrogen atom (e.g., methyl - CH<sub>3</sub>, ethyl - C<sub>2</sub>H<sub>5</sub>, propyl - C<sub>3</sub>H<sub>7</sub>, butyl - C<sub>4</sub>H<sub>9</sub>).
- Haloalkanes: These contain halogen atoms (F, Cl, Br, I) as substituents (e.g., fluoro-, chloro-, bromo-, iodo-).
- Other functional groups: These include alcohols (-OH), aldehydes (-CHO), ketones (-CO-), carboxylic acids (-COOH), amines (-NH<sub>2</sub>), and many others. These functional groups often dictate the suffix used in the IUPAC name.
Putting it All Together: Constructing the IUPAC Name
The IUPAC name is constructed by combining the names of the substituents (in alphabetical order, ignoring prefixes like di-, tri-) with the name of the parent alkane. The position of the substituents is indicated by the number assigned to the carbon atom to which they are attached.
Example:
Let's name the following compound: CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>
- Identify the parent chain: The longest continuous carbon chain contains five carbon atoms, making it pentane.
- Number the carbon atoms: Numbering from either end gives the same result: the methyl group is on carbon 2.
- Identify and name the substituent: The substituent is a methyl group.
- Construct the IUPAC name: The IUPAC name is 2-methylpentane.
Naming Alkanes, Alkenes, and Alkynes
Alkanes, alkenes, and alkynes form the foundation of organic chemistry. Their IUPAC names are built upon the length of their carbon chain and the type of bonds present.
Alkanes
Alkanes are hydrocarbons containing only single bonds. Their IUPAC names use the prefix indicating the number of carbons followed by "-ane."
| Number of Carbons | Prefix | IUPAC Name |
|---|---|---|
| 1 | Meth- | Methane |
| 2 | Eth- | Ethane |
| 3 | Prop- | Propane |
| 4 | But- | Butane |
| 5 | Pent- | Pentane |
| 6 | Hex- | Hexane |
| 7 | Hept- | Heptane |
| 8 | Oct- | Octane |
| 9 | Non- | Nonane |
| 10 | Dec- | Decane |
Alkenes
Alkenes contain at least one carbon-carbon double bond. Their IUPAC names use the same prefixes as alkanes but with the suffix "-ene." The position of the double bond is indicated by the lower number of the two carbons involved in the double bond.
Example: CH<sub>2</sub>=CHCH<sub>2</sub>CH<sub>3</sub> is named 1-butene.
Alkynes
Alkynes contain at least one carbon-carbon triple bond. Their IUPAC names use the same prefixes as alkanes, but with the suffix "-yne." The position of the triple bond is indicated similarly to alkenes.
Example: CH≡CCH<sub>2</sub>CH<sub>3</sub> is named 1-butyne.
Naming Compounds with Multiple Functional Groups
When a compound contains multiple functional groups, a priority order is followed to determine which group dictates the suffix and which groups are treated as substituents. The priority order generally follows the order of decreasing oxidation state. Carboxylic acids have the highest priority, followed by aldehydes, ketones, alcohols, amines, and so on.
Alcohols
Alcohols contain the hydroxyl group (-OH). The IUPAC name is derived from the parent alkane, replacing the "-e" ending with "-ol". The position of the hydroxyl group is indicated by a number.
Example: CH<sub>3</sub>CH(OH)CH<sub>3</sub> is named 2-propanol.
Aldehydes
Aldehydes contain the formyl group (-CHO). The IUPAC name is derived from the parent alkane, replacing the "-e" ending with "-al." The aldehyde group is always on carbon 1, so its position doesn't need to be specified.
Example: CH<sub>3</sub>CH<sub>2</sub>CHO is named propanal.
Ketones
Ketones contain the carbonyl group (=O) bonded to two carbon atoms. The IUPAC name is derived from the parent alkane, replacing the "-e" ending with "-one." The position of the carbonyl group is indicated by a number.
Example: CH<sub>3</sub>COCH<sub>3</sub> is named propanone (also known as acetone).
Carboxylic Acids
Carboxylic acids contain the carboxyl group (-COOH). The IUPAC name is derived from the parent alkane, replacing the "-e" ending with "-oic acid." The carboxyl group is always on carbon 1, so its position doesn't need to be specified.
Example: CH<sub>3</sub>COOH is named ethanoic acid (also known as acetic acid).
Handling Complex Molecules: Multiple Substituents and Branching
When dealing with molecules containing multiple substituents or significant branching, the process becomes more intricate but remains systematic. Remember the following key steps:
- Identify the longest continuous carbon chain: This forms the basis of the parent name.
- Number the carbon atoms: Assign numbers to carbons in the main chain to give substituents the lowest possible numbers.
- Identify and name all substituents: Use appropriate prefixes (di-, tri-, tetra-, etc.) to indicate the number of identical substituents.
- Arrange substituents alphabetically: List substituents alphabetically before the parent name, ignoring prefixes like di-, tri-.
- Combine to create the IUPAC name: Include the location numbers of the substituents before their names.
Example: Consider a molecule with a parent chain of hexane and the following substituents: 2 methyl groups on carbons 2 and 4, and an ethyl group on carbon 3.
The IUPAC name would be: 3-ethyl-2,4-dimethylhexane
Beyond the Basics: Cycloalkanes and Aromatic Compounds
The principles discussed above extend to more complex structures like cycloalkanes and aromatic compounds.
Cycloalkanes
Cycloalkanes are saturated hydrocarbons with carbon atoms arranged in a ring. The prefix "cyclo-" is added before the alkane name corresponding to the number of carbon atoms in the ring.
Example: A cycloalkane with three carbon atoms is named cyclopropane.
Aromatic Compounds
Aromatic compounds, such as benzene derivatives, have specific naming conventions based on their structure and substituents. Benzene derivatives often use common names along with IUPAC names.
Example: Methylbenzene is commonly known as toluene.
Conclusion
Mastering IUPAC nomenclature requires practice and attention to detail. By systematically following the rules outlined above, you can confidently name a wide variety of organic compounds. Remember to practice regularly with various examples to reinforce your understanding. The ability to accurately name and understand the nomenclature of organic compounds is an essential skill for any chemist. This comprehensive guide provides a solid foundation for navigating the intricacies of this system, equipping you to confidently name and interpret organic structures. By understanding the underlying principles, you can apply this knowledge to even more complex molecules as your expertise grows. Remember to consult resources and practice frequently to solidify your understanding of this crucial aspect of organic chemistry.
Latest Posts
Latest Posts
-
Which Of The Following Is Polynomial
Mar 17, 2025
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Give The Iupac Name For The Following Compound. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
