Freezing Point Of Water On Celsius Scale
News Leon
Mar 20, 2025 · 5 min read
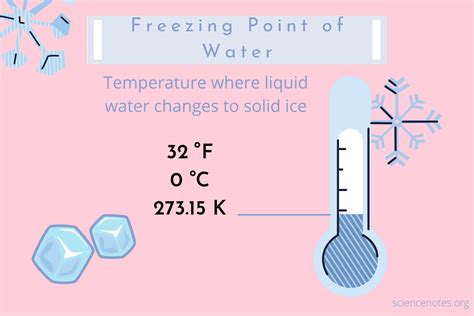
Table of Contents
The Freezing Point of Water: A Deep Dive into 0° Celsius
The freezing point of water, a seemingly simple concept, holds significant importance across various scientific disciplines and everyday life. Understanding this fundamental property of water is crucial for numerous applications, from weather forecasting and industrial processes to biological functions and culinary arts. This article will explore the freezing point of water on the Celsius scale (0°C), delving into its scientific basis, factors influencing it, and its wider implications.
Understanding the Freezing Point
The freezing point of a substance is the temperature at which it transitions from a liquid state to a solid state. For pure water under standard atmospheric pressure (1 atmosphere or 101.325 kPa), this transition occurs at precisely 0° Celsius (0°C), which is equivalent to 32° Fahrenheit (32°F) and 273.15 Kelvin (273.15 K). This temperature represents the point where the kinetic energy of water molecules decreases sufficiently for the intermolecular forces (hydrogen bonds) to overcome their movement and form a stable crystalline structure – ice.
The Role of Hydrogen Bonding
Water's unique properties, including its relatively high freezing point compared to other similar-sized molecules, are largely attributed to the presence of hydrogen bonds. These strong intermolecular forces exist between the slightly positive hydrogen atom of one water molecule and the slightly negative oxygen atom of another. These bonds create a relatively strong cohesive structure within liquid water. As the temperature drops towards 0°C, the kinetic energy of the water molecules diminishes, allowing the hydrogen bonds to effectively hold the molecules in place, forming the rigid, ordered structure of ice.
Factors Affecting the Freezing Point of Water
While 0°C is the standard freezing point of water, several factors can influence this temperature, causing it to deviate from this value.
Pressure
Pressure plays a significant role in determining the freezing point of water. Increasing the pressure on water actually lowers its freezing point. This is an unusual property, unlike most substances where increased pressure raises the freezing point. This anomalous behavior is related to the density difference between liquid water and ice. Ice is less dense than liquid water, a phenomenon resulting from the crystalline structure of ice that allows for more space between water molecules. Therefore, increasing the pressure favors the denser liquid phase, lowering the freezing point.
Impurities and Dissolved Substances
The presence of impurities or dissolved substances in water also affects its freezing point. This phenomenon is known as freezing point depression. The addition of solutes, such as salt or sugar, disrupts the formation of the ice crystal lattice, requiring a lower temperature for freezing to occur. This principle is applied in various applications, such as de-icing roads and sidewalks during winter. The salt lowers the freezing point of water, preventing ice formation even at sub-zero temperatures.
Solute Concentration
The magnitude of freezing point depression is directly proportional to the concentration of the dissolved solute. A higher concentration of solute leads to a greater decrease in the freezing point. This relationship is described by the colligative properties of solutions, which depend on the number of solute particles present rather than their identity.
Supercooling
Sometimes, water can be cooled below 0°C without freezing. This phenomenon is called supercooling. It occurs when there are insufficient nucleation sites – microscopic irregularities or impurities – for ice crystals to begin forming. In the absence of these sites, the water molecules lack the necessary structure to initiate crystallization even at temperatures below the freezing point. However, a slight disturbance, such as shaking the container or adding an ice crystal, can trigger immediate freezing.
Practical Applications of the Freezing Point of Water
The knowledge of water's freezing point is fundamental in numerous fields:
Meteorology and Climate Science
Understanding the freezing point of water is essential for weather forecasting and climate science. The freezing of water in the atmosphere contributes to cloud formation, precipitation, and the overall weather patterns. Variations in temperature and pressure affect the formation of ice crystals in clouds, impacting the type and intensity of precipitation.
Biology and Medicine
The freezing point of water is crucial for biological processes. Many organisms have evolved strategies to cope with freezing temperatures, employing various mechanisms to protect their cells from ice crystal damage. In medicine, freezing is used for cryopreservation, the preservation of cells, tissues, and organs at low temperatures. Understanding how different solutes affect the freezing point is important to minimize damage during cryopreservation.
Food Science and Culinary Arts
The freezing point of water directly impacts food preservation and culinary techniques. Freezing food at 0°C or below inhibits the growth of microorganisms, extending its shelf life. Many culinary techniques also depend on the freezing and melting of water, such as creating ice cream or preparing certain desserts.
Industrial Processes
Numerous industrial processes rely on the freezing point of water. The freezing and thawing of water can be utilized in various manufacturing techniques, including the creation of certain materials and the separation of substances.
The Importance of Accuracy in Measuring the Freezing Point
Accurate measurement of the freezing point of water is crucial for scientific research and industrial applications. The slightest deviation from 0°C under standard conditions could indicate impurities or variations in pressure, impacting experimental results or industrial processes. Therefore, precise and calibrated instruments are essential for accurate measurements.
Calibration and Instrumentation
Specialized instruments, such as thermometers with high accuracy and precision, are utilized to measure the freezing point of water. Regular calibration of these instruments against known standards ensures accurate readings. The accuracy of the freezing point measurement is dependent on the precision of the thermometer used, the purity of the water sample, and the control of environmental factors such as pressure.
Conclusion
The freezing point of water at 0°C on the Celsius scale is a fundamental physical property with far-reaching implications across diverse scientific disciplines and practical applications. Understanding the factors that can influence this point – pressure, impurities, and supercooling – is crucial for accurate measurements and informed decision-making in various fields. From weather forecasting and biological processes to industrial applications and culinary arts, the significance of this seemingly simple concept cannot be overstated. Ongoing research continues to deepen our understanding of the behavior of water at its freezing point, leading to advancements in many areas of science and technology. This intricate relationship between temperature, pressure, and the molecular structure of water showcases the profound complexity and essential role this substance plays in shaping our world.
Latest Posts
Latest Posts
-
What Percent Of 120 Is 42
Mar 20, 2025
-
What Is The Electron Configuration Of Aluminum
Mar 20, 2025
-
A Complete Virus Particle Is Called A
Mar 20, 2025
-
Which Of The Following Is Not A Protein Function
Mar 20, 2025
-
Explain How Ionic Compounds Dissolve In Water
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point Of Water On Celsius Scale . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
