Explain How Ionic Compounds Dissolve In Water
News Leon
Mar 20, 2025 · 6 min read
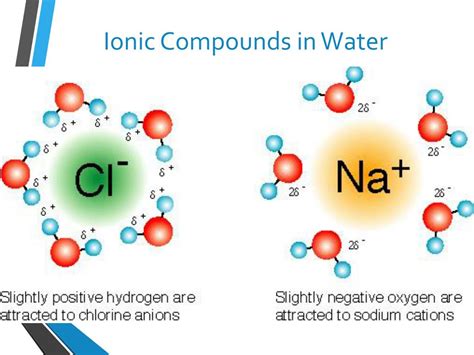
Table of Contents
How Ionic Compounds Dissolve in Water: A Deep Dive
Understanding how ionic compounds dissolve in water is crucial for grasping many fundamental concepts in chemistry and beyond. From biological processes to industrial applications, the solubility of ionic compounds in water plays a vital role. This in-depth article will explore the intricate mechanism behind this process, demystifying the seemingly simple act of dissolving salt in water.
The Nature of Ionic Compounds
Before delving into the dissolution process, let's establish a firm understanding of ionic compounds themselves. Ionic compounds are formed through the electrostatic attraction between oppositely charged ions. These ions are created when atoms transfer electrons, leading to the formation of cations (positively charged ions) and anions (negatively charged ions). The strong electrostatic forces holding these ions together create a crystalline lattice structure, a highly ordered arrangement of ions. Common examples include sodium chloride (NaCl, table salt), potassium chloride (KCl), and calcium carbonate (CaCO₃).
Strong Electrostatic Forces: The Key to Crystalline Structure
The strength of the electrostatic attraction within the ionic lattice depends on several factors, primarily:
- Charge magnitude: Higher charges on the ions lead to stronger attraction. For example, the attraction between Mg²⁺ and O²⁻ is stronger than that between Na⁺ and Cl⁻.
- Ionic radii: Smaller ions result in stronger attraction because the charges are closer together.
The crystalline structure is incredibly stable, requiring significant energy to break the bonds holding it together. This is why ionic compounds typically exist as solids at room temperature.
The Role of Water: A Polar Solvent
Water (H₂O) is a unique and essential solvent due to its polar nature. The oxygen atom in water is more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly. This creates a polar molecule with a slightly negative charge (δ-) on the oxygen atom and slightly positive charges (δ+) on the hydrogen atoms. This uneven charge distribution is what makes water such an effective solvent for many ionic compounds.
Water's Dipole Moment: The Driving Force
The polarity of water results in a dipole moment, a measure of the separation of positive and negative charges within the molecule. This dipole moment is the key to understanding how water interacts with and dissolves ionic compounds. The slightly positive hydrogen atoms of water molecules are attracted to the negatively charged anions in the ionic lattice, while the slightly negative oxygen atoms are attracted to the positively charged cations.
The Dissolution Process: Step-by-Step
The dissolution of an ionic compound in water is a dynamic equilibrium process. It involves several key steps:
-
Water molecules approach the ionic lattice: As water molecules come into contact with the surface of the ionic crystal, their dipole moments interact with the ions at the surface.
-
Hydration of ions: The slightly positive hydrogen atoms of water molecules surround and interact with the anions, while the slightly negative oxygen atoms surround and interact with the cations. This process is called hydration, and it weakens the electrostatic forces holding the ions together in the lattice. The water molecules effectively shield the ions from each other, reducing the attractive forces.
-
Breaking of ionic bonds: As more water molecules surround the ions, the electrostatic forces within the ionic lattice are further weakened. Eventually, the attractive forces between the ions and the water molecules overcome the attractive forces within the ionic lattice. This leads to the ions breaking away from the crystal structure.
-
Dispersion of ions: Once separated from the lattice, the hydrated ions are dispersed throughout the water, becoming surrounded by a shell of water molecules. These hydrated ions are now free to move independently in solution.
-
Equilibrium: The dissolution process continues until a state of dynamic equilibrium is reached. At equilibrium, the rate at which ions leave the crystal lattice is equal to the rate at which hydrated ions return to the lattice and re-form the crystal. The concentration of dissolved ions at equilibrium is known as the solubility of the ionic compound in water.
Factors Affecting Solubility
Several factors influence the solubility of ionic compounds in water:
-
Lattice energy: The strength of the electrostatic forces within the ionic lattice. Higher lattice energy means lower solubility.
-
Hydration energy: The energy released when ions are hydrated. Higher hydration energy means higher solubility. The size and charge of the ions significantly influence hydration energy. Smaller ions with higher charges have stronger hydration interactions.
-
Temperature: The solubility of most ionic compounds increases with increasing temperature. This is because the increased kinetic energy of the water molecules helps to overcome the lattice energy. However, there are exceptions to this rule.
-
Pressure: Pressure has a negligible effect on the solubility of ionic compounds in water.
Applications and Real-World Examples
The solubility of ionic compounds in water is crucial in numerous applications:
-
Biological systems: Many biological processes rely on the solubility of ions in water. For example, the transport of nutrients and electrolytes in the human body depends on the ability of ions to dissolve in blood plasma.
-
Medicine: Many medications are formulated as ionic compounds to improve their solubility and absorption in the body.
-
Industrial processes: The solubility of ionic compounds is crucial in many industrial processes, such as the production of fertilizers, detergents, and other chemicals.
-
Environmental science: Understanding the solubility of ionic compounds is important for assessing the environmental impact of pollutants and for developing effective water treatment methods.
Beyond Simple Dissolution: Factors Affecting Equilibrium
While the description above provides a fundamental understanding, the reality of ionic compound dissolution is far more nuanced. Several additional factors contribute to the overall solubility and behavior of these compounds in water:
-
Common ion effect: The presence of a common ion in solution reduces the solubility of a slightly soluble ionic compound.
-
Complex ion formation: The formation of complex ions can significantly affect solubility. Certain ions can coordinate with the ions of the ionic compound, leading to an increase in solubility.
-
pH effects: The pH of the solution can influence the solubility of certain ionic compounds, particularly those containing weak acids or bases.
-
Presence of other solutes: The presence of other solutes in the water can affect the solubility of an ionic compound through interactions like ion pairing and changes in ionic strength.
Conclusion
The dissolution of ionic compounds in water is a complex yet fascinating process governed by the interplay of electrostatic forces, hydration energies, and various environmental factors. Understanding this process is crucial for numerous scientific disciplines and practical applications. This detailed explanation serves as a foundation for further exploration of the intricate world of solubility and its implications across various fields. By understanding the fundamental principles, we can better appreciate the significance of this seemingly simple phenomenon and its profound impact on our world.
Latest Posts
Latest Posts
-
What Is Half Of 1 1 2 Tsp
Mar 21, 2025
-
Which Is Not A Cranial Bone
Mar 21, 2025
-
What Type Of Symmetry Do Echinoderms Have
Mar 21, 2025
-
90 Is What Percent Of 120
Mar 21, 2025
-
How Many Cubic Meters In A Cubic Centimeter
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Explain How Ionic Compounds Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
