Freezing Point Depression Constant Of Water
News Leon
Mar 16, 2025 · 6 min read
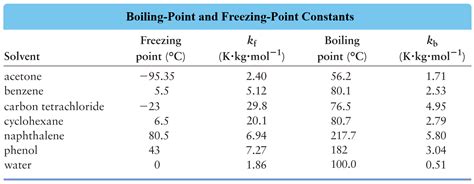
Table of Contents
Freezing Point Depression Constant of Water: A Deep Dive
The freezing point depression constant, often denoted as K<sub>f</sub>, is a fundamental colligative property of solvents. It quantifies the extent to which the freezing point of a solvent is lowered when a solute is added. This phenomenon, known as freezing point depression, is crucial in various applications, from de-icing roads to understanding biological processes. This article will delve deep into the freezing point depression constant of water, exploring its definition, calculation, applications, and factors influencing its value.
Understanding Freezing Point Depression
Before diving into the specifics of water's K<sub>f</sub>, let's establish a foundational understanding of freezing point depression. When a non-volatile solute is added to a solvent, the freezing point of the resulting solution is lower than that of the pure solvent. This is because the solute particles disrupt the solvent's crystal lattice structure, making it more difficult for the solvent molecules to arrange themselves into the ordered solid state. The greater the concentration of solute particles, the greater the depression in the freezing point.
Colligative Properties: A Key Concept
Freezing point depression is a colligative property, meaning it depends on the number of solute particles present in the solution, not their identity or chemical nature. Other colligative properties include boiling point elevation, osmotic pressure, and vapor pressure lowering. This is why the freezing point depression constant is a crucial parameter for understanding the behavior of solutions.
The Freezing Point Depression Constant (K<sub>f</sub>) of Water
The freezing point depression constant of water, K<sub>f</sub>, is 1.86 °C kg/mol. This means that a 1 molal (1 mol solute per kilogram of water) solution of a non-electrolyte will have its freezing point lowered by 1.86 °C compared to pure water (which freezes at 0 °C). It's crucial to understand that this value applies specifically to water as the solvent; different solvents will have different K<sub>f</sub> values.
The Equation: ΔT<sub>f</sub> = K<sub>f</sub> * m * i
The magnitude of freezing point depression (ΔT<sub>f</sub>) can be calculated using the following equation:
ΔT<sub>f</sub> = K<sub>f</sub> * m * i
Where:
- ΔT<sub>f</sub> is the change in freezing point (in °C)
- K<sub>f</sub> is the freezing point depression constant (1.86 °C kg/mol for water)
- m is the molality of the solution (moles of solute per kilogram of solvent)
- i is the van't Hoff factor, representing the number of particles a solute dissociates into in solution. For non-electrolytes (like sugar), i = 1. For strong electrolytes (like NaCl), i is greater than 1 (2 for NaCl, considering complete dissociation).
Calculating Freezing Point Depression: Worked Examples
Let's illustrate the application of the freezing point depression equation with a couple of examples:
Example 1: Non-electrolyte
Calculate the freezing point of a solution containing 10 grams of glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>, molar mass = 180.16 g/mol) dissolved in 100 grams of water.
-
Calculate molality (m):
- Moles of glucose = (10 g) / (180.16 g/mol) = 0.0555 mol
- Mass of water = 100 g = 0.1 kg
- Molality (m) = 0.0555 mol / 0.1 kg = 0.555 mol/kg
-
Apply the equation:
- ΔT<sub>f</sub> = K<sub>f</sub> * m * i = (1.86 °C kg/mol) * (0.555 mol/kg) * (1) = 1.03 °C
-
Determine the freezing point:
- Freezing point = 0 °C - 1.03 °C = -1.03 °C
Example 2: Strong Electrolyte
Calculate the freezing point of a solution containing 5.85 grams of sodium chloride (NaCl, molar mass = 58.44 g/mol) dissolved in 250 grams of water.
-
Calculate molality (m):
- Moles of NaCl = (5.85 g) / (58.44 g/mol) = 0.1 mol
- Mass of water = 250 g = 0.25 kg
- Molality (m) = 0.1 mol / 0.25 kg = 0.4 mol/kg
-
Apply the equation:
- Since NaCl is a strong electrolyte that dissociates completely into Na<sup>+</sup> and Cl<sup>-</sup> ions, i = 2.
- ΔT<sub>f</sub> = K<sub>f</sub> * m * i = (1.86 °C kg/mol) * (0.4 mol/kg) * (2) = 1.49 °C
-
Determine the freezing point:
- Freezing point = 0 °C - 1.49 °C = -1.49 °C
Factors Affecting the Freezing Point Depression Constant
While the K<sub>f</sub> value for water is generally considered constant at 1.86 °C kg/mol, slight variations can occur due to several factors:
- Purity of water: Impurities in the water can affect its freezing point and, consequently, the observed K<sub>f</sub> value. High-purity water is essential for accurate measurements.
- Pressure: While the effect is minimal at normal pressures, significant changes in pressure can influence the K<sub>f</sub> value.
- Intermolecular interactions: Stronger intermolecular interactions between solute and solvent molecules can slightly alter the extent of freezing point depression.
Applications of Freezing Point Depression
The understanding and application of freezing point depression are widespread across numerous fields:
1. De-icing:
This is perhaps the most familiar application. Adding salts (like NaCl or CaCl<sub>2</sub>) to roads and sidewalks lowers the freezing point of water, preventing ice formation at temperatures below 0 °C. The effectiveness depends on the salt's solubility and its van't Hoff factor.
2. Antifreeze in Automobiles:
Ethylene glycol is commonly used as an antifreeze in car radiators. It lowers the freezing point of the coolant, preventing it from freezing in cold weather and damaging the engine.
3. Food Preservation:
Freezing food involves lowering its temperature below its freezing point, slowing down or stopping microbial growth and enzymatic activity. The principles of freezing point depression are applied in understanding the freezing processes in food preservation.
4. Cryobiology:
Cryobiology deals with the effects of low temperatures on living organisms. Understanding freezing point depression is vital in developing cryopreservation techniques, which involve freezing biological samples (cells, tissues, organs) for long-term storage.
5. Physical Chemistry and Thermodynamics:
Freezing point depression is a valuable tool in determining the molar mass of unknown substances. By measuring the freezing point depression of a solution with a known mass of solute, the molar mass can be calculated. This finds applications in various chemical analyses.
6. Medical Applications:
In medicine, intravenous solutions are carefully formulated to match the osmotic pressure of blood to prevent damage to red blood cells. This concept involves freezing point depression indirectly.
7. Environmental Science:
The study of freezing point depression helps in understanding the behavior of natural water systems, like saline lakes or seawater, where the presence of dissolved salts affects their freezing points.
Conclusion
The freezing point depression constant of water, K<sub>f</sub> = 1.86 °C kg/mol, is a critical parameter in understanding the colligative properties of aqueous solutions. Its applications are widespread, from practical applications like de-icing to sophisticated scientific techniques in cryobiology and molar mass determination. While the value remains relatively constant under standard conditions, factors like purity, pressure, and intermolecular interactions can subtly influence its value. A thorough grasp of this constant and its associated equations is essential for anyone working with solutions or tackling problems involving freezing point depression.
Latest Posts
Latest Posts
-
Which Of The Following Is Polynomial
Mar 17, 2025
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point Depression Constant Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
