Fecl3 Naoh Fe Oh 3 Nacl
News Leon
Mar 28, 2025 · 5 min read
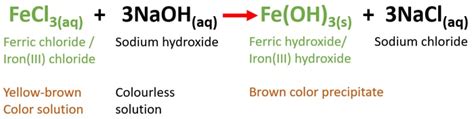
Table of Contents
The Reaction Between FeCl3 and NaOH: A Deep Dive into the Chemistry of Fe(OH)3 and NaCl Formation
The reaction between ferric chloride (FeCl₃) and sodium hydroxide (NaOH) is a classic example of a double displacement reaction, also known as a metathesis reaction. This seemingly simple reaction yields ferric hydroxide (Fe(OH)₃) – a reddish-brown precipitate – and sodium chloride (NaCl), common table salt, dissolved in solution. Understanding this reaction requires delving into the properties of each reactant and product, the stoichiometry of the reaction, and the various applications and implications of this chemical process.
Understanding the Reactants: FeCl₃ and NaOH
Ferric Chloride (FeCl₃): This is an inorganic compound, existing as a dark brown solid or yellowish-brown solution in water. It's highly soluble in water, readily dissociating into its constituent ions, Fe³⁺ (ferric or iron(III) ion) and Cl⁻ (chloride ion). The Fe³⁺ ion is a transition metal ion, exhibiting variable oxidation states and forming a variety of complexes. Its high charge density makes it a strong Lewis acid, meaning it readily accepts electron pairs.
Sodium Hydroxide (NaOH): Also known as caustic soda or lye, NaOH is a strong alkali, readily dissolving in water to form a strongly basic solution. It completely dissociates into Na⁺ (sodium ion) and OH⁻ (hydroxide ion). The hydroxide ion is a strong Brønsted-Lowry base, readily accepting protons (H⁺).
Properties and Characteristics of the Reactants: A Comparative Overview
| Property | FeCl₃ | NaOH |
|---|---|---|
| Chemical Formula | FeCl₃ | NaOH |
| Appearance | Dark brown solid/Yellowish-brown solution | White crystalline solid |
| Solubility in Water | Highly soluble | Highly soluble |
| Acidity/Basicity | Acidic (forms acidic solution in water) | Basic (forms strongly basic solution) |
| Toxicity | Moderately toxic | Highly corrosive and toxic |
| Uses | Water treatment, etching, catalyst | Soap making, paper production, drain cleaner |
The Reaction: FeCl₃ + 3NaOH → Fe(OH)₃ + 3NaCl
The reaction between ferric chloride and sodium hydroxide is an example of a precipitation reaction. When aqueous solutions of FeCl₃ and NaOH are mixed, a double displacement reaction occurs, leading to the formation of ferric hydroxide, Fe(OH)₃, and sodium chloride, NaCl. The balanced chemical equation is:
FeCl₃(aq) + 3NaOH(aq) → Fe(OH)₃(s) + 3NaCl(aq)
The (aq) indicates that the substance is dissolved in water (aqueous solution), while (s) denotes a solid precipitate. Note the stoichiometric coefficients: one mole of FeCl₃ reacts with three moles of NaOH to produce one mole of Fe(OH)₃ and three moles of NaCl. This ratio is crucial for accurate calculations involving the reaction.
Understanding the Products: Fe(OH)₃ and NaCl
Ferric Hydroxide (Fe(OH)₃): This is the reddish-brown precipitate formed in the reaction. It's an insoluble compound, meaning it doesn't dissolve significantly in water. Fe(OH)₃ is an important compound in various applications, including water treatment and the production of other iron compounds. It's amphoteric, meaning it can react with both acids and bases.
Sodium Chloride (NaCl): This is common table salt, a highly soluble ionic compound. It completely dissociates in water into Na⁺ and Cl⁻ ions. NaCl is a crucial electrolyte in biological systems and has numerous industrial applications.
Properties and Characteristics of the Products: A Comparative Overview
| Property | Fe(OH)₃ | NaCl |
|---|---|---|
| Chemical Formula | Fe(OH)₃ | NaCl |
| Appearance | Reddish-brown precipitate | White crystalline solid |
| Solubility in Water | Insoluble | Highly soluble |
| Acidity/Basicity | Amphoteric | Neutral (solution is neutral) |
| Toxicity | Low toxicity | Low toxicity at typical concentrations |
| Uses | Water treatment, pigment production | Food preservation, de-icing, industrial uses |
The Precipitation Process: A Closer Look
The formation of the Fe(OH)₃ precipitate is driven by the low solubility product constant (Ksp) of Fe(OH)₃. The Ksp is an equilibrium constant that represents the extent to which a sparingly soluble salt will dissolve in water. A low Ksp indicates that the compound is largely insoluble. When the concentrations of Fe³⁺ and OH⁻ ions exceed the solubility product, Fe(OH)₃ precipitates out of the solution. This process is influenced by factors like temperature and the presence of other ions.
Applications and Implications
This reaction has several practical applications:
-
Water Treatment: Fe(OH)₃ is used as a flocculant in water treatment plants. Its precipitation helps remove suspended impurities and other contaminants from water.
-
Pigment Production: Fe(OH)₃ can be further processed to produce iron oxides, which are used as pigments in paints and other materials.
-
Chemical Synthesis: The reaction serves as a starting point for the synthesis of various iron-containing compounds.
-
Laboratory Experiments: It's a common demonstration reaction in chemistry education, illustrating concepts like double displacement reactions, precipitation, and stoichiometry.
Safety Precautions
Both FeCl₃ and NaOH are corrosive and can cause skin and eye irritation. Always handle these chemicals with appropriate safety precautions, including wearing gloves, eye protection, and a lab coat. In case of contact, immediately rinse the affected area with plenty of water. Proper disposal of the waste products is also crucial.
Further Exploration: Advanced Concepts
-
Solubility Equilibria: Exploring the concept of solubility product constant (Ksp) and its influence on the precipitation of Fe(OH)₃.
-
Complex Ion Formation: Investigating the formation of complex ions involving Fe³⁺ and other ligands.
-
Kinetics of the Reaction: Studying the rate at which the reaction proceeds under different conditions.
-
Spectroscopic Analysis: Utilizing techniques like UV-Vis spectroscopy to monitor the reaction progress and quantify the concentrations of reactants and products.
Conclusion: A Fundamental Reaction with Diverse Applications
The reaction between FeCl₃ and NaOH, resulting in the formation of Fe(OH)₃ and NaCl, is a fundamental chemical process with widespread applications. Understanding the properties of each reactant and product, the stoichiometry of the reaction, and the underlying principles of precipitation is crucial for various scientific and industrial applications. Further exploration of related concepts, like solubility equilibria and complex ion formation, offers a deeper understanding of this seemingly simple yet impactful chemical reaction. This comprehensive overview provides a solid foundation for further studies in chemistry and related fields. The detailed explanation of reactants, products, and the reaction mechanism allows for a thorough grasp of the chemical processes involved. The inclusion of safety precautions and further exploration sections enhances the article's completeness and reader engagement.
Latest Posts
Latest Posts
-
Which Of The Following Is The Most Stable Cation
Mar 31, 2025
-
Who Is The Father Of Bio
Mar 31, 2025
-
Which Of The Following Is True About Genes
Mar 31, 2025
-
What Animal Has The Largest Breasts
Mar 31, 2025
-
Which Two Bonds Are Most Similar In Polarity
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Fecl3 Naoh Fe Oh 3 Nacl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
