Which Of The Following Is The Most Stable Cation
News Leon
Mar 31, 2025 · 6 min read
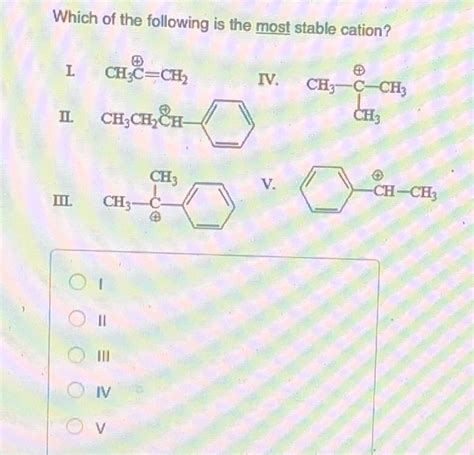
Table of Contents
Which of the Following is the Most Stable Cation? A Deep Dive into Cation Stability
Determining the most stable cation among a given set requires a nuanced understanding of several key factors influencing cationic stability. This isn't a simple matter of comparing charges; it's about considering the interplay of charge density, size, electronic configuration, and polarizability. This article will explore these factors in detail, providing a robust framework for assessing cation stability and addressing the question posed in various contexts.
Understanding Cation Stability: The Fundamental Principles
A cation is a positively charged ion, formed when an atom loses one or more electrons. The stability of a cation is inversely related to its tendency to regain those lost electrons. A more stable cation is less reactive and less likely to participate in chemical reactions involving electron transfer. Several factors contribute to a cation's overall stability:
1. Charge Density: This refers to the ratio of the cation's charge to its size (ionic radius). A higher charge density indicates a greater concentration of positive charge in a smaller volume. High charge density leads to stronger electrostatic attraction between the cation and surrounding anions or electron-rich species, increasing reactivity. Conversely, a lower charge density results in weaker interactions, leading to greater stability.
2. Ionic Radius: Smaller cations generally have higher charge densities and are less stable than larger cations with the same charge. The smaller size leads to a greater concentration of positive charge, attracting electrons more strongly. Larger ions, on the other hand, have their charge spread over a larger volume, reducing the effective charge density.
3. Electronic Configuration: Cations with noble gas electronic configurations (ns²np⁶) are exceptionally stable. These configurations represent a filled electron shell, providing maximum stability due to electron pairing and the absence of readily available electrons for reaction. Cations that don't achieve a noble gas configuration are less stable, and their reactivity depends on the extent of deviation from this ideal state.
4. Polarizability: This refers to the ease with which the electron cloud of an ion can be distorted by an external electric field. Larger ions are generally more polarizable than smaller ones. High polarizability can lead to increased reactivity as the electron cloud's distortion facilitates interactions with other species.
Comparing Cation Stability: A Case-by-Case Analysis
To illustrate the principles discussed, let's consider a hypothetical comparison of several cations: Li⁺, Na⁺, K⁺, Mg²⁺, and Al³⁺. We will analyze their stability based on the factors outlined above.
1. Alkali Metal Cations (Li⁺, Na⁺, K⁺):
-
Li⁺: Lithium possesses the smallest ionic radius among the alkali metals. This results in a high charge density, making Li⁺ relatively less stable compared to its heavier counterparts. It readily participates in reactions involving electron transfer.
-
Na⁺: Sodium has a larger ionic radius than lithium, leading to a lower charge density and increased stability compared to Li⁺. However, it's still smaller than potassium, resulting in moderate stability.
-
K⁺: Potassium possesses the largest ionic radius among this group, translating into the lowest charge density and therefore the highest stability among the three alkali metal cations. It exhibits lower reactivity compared to Li⁺ and Na⁺.
Trend: Within the alkali metals, stability increases down the group due to the increasing ionic radius and decreasing charge density.
2. Alkaline Earth Metal Cations (Mg²⁺, Ca²⁺):
-
Mg²⁺: Magnesium, with a +2 charge, has a significantly higher charge density than the alkali metals discussed previously. This higher charge makes it more reactive and less stable than the alkali metal cations. The smaller size further contributes to its instability.
-
Ca²⁺: Calcium, with a larger ionic radius than magnesium, possesses a lower charge density, making it slightly more stable than Mg²⁺. However, the +2 charge still contributes to significant reactivity compared to the alkali metals.
3. Aluminum Cation (Al³⁺):
- Al³⁺: Aluminum, with a +3 charge, has an extremely high charge density. This makes Al³⁺ one of the least stable cations among the group. Its small size intensifies the effect of the high charge, rendering it highly reactive.
Overall Comparison and Conclusion:
Considering all the factors – charge density, ionic radius, electronic configuration, and polarizability – K⁺ emerges as the most stable cation among the group Li⁺, Na⁺, K⁺, Mg²⁺, and Al³⁺. Its larger ionic radius and +1 charge lead to a significantly lower charge density compared to the others. Moreover, all these ions have a stable noble gas electron configuration.
Expanding the Analysis: Beyond Simple Cations
The principles discussed above can be applied to a broader range of cations. However, other factors can significantly influence stability in more complex scenarios:
-
Ligand Effects: The presence of ligands (ions or molecules bound to the cation) can significantly alter its stability. Ligands can reduce the effective charge density through electron donation, shielding the cation from external interactions. The nature of the ligand (size, charge, electron-donating ability) plays a crucial role in determining the extent of stabilization.
-
Coordination Number: The number of ligands surrounding a cation (coordination number) influences its stability. Higher coordination numbers generally lead to increased stability due to greater electrostatic interaction with the ligands.
-
Crystal Field Effects: In solid-state systems, the crystal field environment surrounding the cation can significantly affect its stability. The symmetry and strength of the crystal field can influence the energy levels of the cation's electrons, impacting its reactivity.
-
Relativistic Effects: For heavier elements, relativistic effects can influence the size and energy levels of the electrons, ultimately affecting cation stability. These effects become increasingly important for elements with high atomic numbers.
Practical Applications and Further Considerations
Understanding cation stability is crucial in various fields:
- Chemistry: Predicting the reactivity of different ions in chemical reactions.
- Materials Science: Designing materials with desired properties by choosing cations with appropriate stability.
- Biology: Understanding the role of metal ions in biological systems, where cation stability influences enzymatic activity and other biological processes.
- Geochemistry: Analyzing the behavior of cations in geological environments, where stability influences mineral formation and weathering processes.
The stability of a cation is not an absolute property but rather depends on the specific environment and the interactions it undergoes. This article has provided a fundamental framework for understanding the factors influencing cation stability. Further exploration into specific systems and the application of advanced computational techniques can provide more detailed insights into this crucial aspect of ionic chemistry. Remember that this analysis is a simplified model, and real-world systems often present more complex interactions. However, the principles discussed here provide a strong foundation for understanding the relative stability of different cations.
Latest Posts
Latest Posts
-
What Bone Articulates With The Acetabulum
Apr 01, 2025
-
What Is The Value Of Log Subscript 27 Baseline 9
Apr 01, 2025
-
How Many Chromosomes In Liver Cells
Apr 01, 2025
-
All Of The Following Refer To Mitosis Except
Apr 01, 2025
-
Which Of The Following Temperatures Is The Coldest
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is The Most Stable Cation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
