Which Two Bonds Are Most Similar In Polarity
News Leon
Mar 31, 2025 · 5 min read
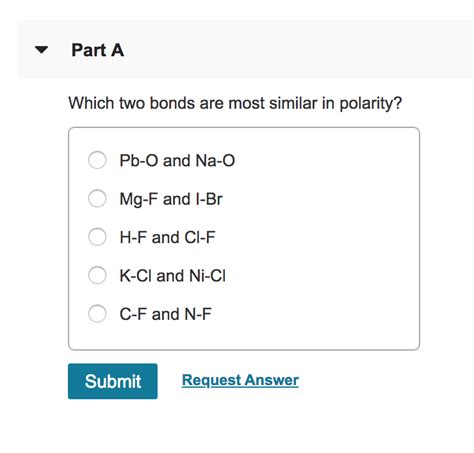
Table of Contents
Which Two Bonds Are Most Similar in Polarity? A Deep Dive into Electronegativity and Bond Character
Determining the similarity in polarity between chemical bonds requires a nuanced understanding of electronegativity and the resulting bond character. While no two bonds will ever be perfectly identical in polarity, we can identify pairs that exhibit very similar degrees of polarity based on the electronegativity difference between the constituent atoms. This article will explore the concept of electronegativity, its influence on bond polarity, and then delve into identifying bond pairs with the most similar polarity. We will also examine the factors that can influence these comparisons and discuss the limitations of simplistic approaches.
Understanding Electronegativity and Bond Polarity
Electronegativity is a fundamental concept in chemistry that describes an atom's ability to attract electrons towards itself within a chemical bond. The higher the electronegativity value, the stronger the atom's pull on shared electrons. This property is determined by various factors, including the atom's effective nuclear charge (the net positive charge experienced by valence electrons) and its atomic radius. Elements on the right side of the periodic table (excluding noble gases) tend to have higher electronegativity values than those on the left.
Bond polarity is a direct consequence of the electronegativity difference between the atoms forming the bond. When two atoms with significantly different electronegativities bond, the more electronegative atom attracts the shared electrons more strongly, creating a polar covalent bond. This uneven distribution of electron density leads to a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom.
Conversely, when two atoms with similar electronegativities bond, the electrons are shared relatively equally, resulting in a nonpolar covalent bond. A purely nonpolar covalent bond only exists when identical atoms bond, such as in diatomic molecules like O₂ or Cl₂. In reality, even bonds between atoms of similar electronegativity exhibit some degree of polarity, although it is minimal.
The Electronegativity Scale: A Tool for Comparison
Various electronegativity scales exist, with the Pauling scale being the most widely used. This scale assigns values to elements, with fluorine (F) having the highest electronegativity (4.0) and francium (Fr) having one of the lowest (0.7). The difference in electronegativity (ΔEN) between two atoms is crucial for predicting the bond's polarity:
- ΔEN = 0: Nonpolar covalent bond (ideally, identical atoms)
- 0 < ΔEN < 0.5: Essentially nonpolar covalent bond; very slight polarity.
- 0.5 < ΔEN < 1.7: Polar covalent bond.
- ΔEN > 1.7: Ionic bond (significant transfer of electrons, not sharing).
Identifying Bond Pairs with Similar Polarity
Pinpointing the two most similar bonds in polarity is inherently challenging because the degree of polarity exists on a spectrum. However, we can identify pairs that exhibit very similar ΔEN values. This requires examining bonds formed between elements with similar electronegativity differences. It's important to note that these comparisons are approximations and are sensitive to the electronegativity scale used.
To illustrate, let's consider some examples:
1. C-C and Si-Si Bonds: Carbon (C) and silicon (Si) are both in Group 14 of the periodic table and exhibit relatively similar electronegativities. The ΔEN for both C-C and Si-Si bonds is very close to zero, resulting in bonds that are essentially nonpolar.
2. C-H and Si-H Bonds: The electronegativity difference between carbon and hydrogen (H) and between silicon and hydrogen is quite small. These bonds are considered relatively nonpolar, with the C-H bond slightly more polar than the Si-H bond due to the higher electronegativity of carbon compared to silicon. These two are often cited as good candidates for similar polarity due to their relative nonpolarity and the proximity of their electronegativity differences.
3. P-Cl and S-Cl Bonds: Phosphorus (P), sulfur (S), and chlorine (Cl) are all relatively close in terms of their electronegativity. Both P-Cl and S-Cl bonds are polar covalent bonds, with ΔEN values relatively close to each other. However, the difference is larger than in the previous examples.
4. N-O and P-O Bonds: Both bonds are polar covalent. The electronegativity differences are relatively similar, falling within a range that indicates moderately polar bonds. Nitrogen and Phosphorus are in the same group, while Oxygen is quite electronegative. This makes these comparisons more complex.
Choosing the "Most Similar": A Critical Analysis
Deciding definitively on the two most similar bonds in polarity is difficult because:
- Different Electronegativity Scales: Different scales yield slightly different electronegativity values, thus influencing ΔEN calculations.
- Context Matters: The bond's environment within a molecule can slightly alter the electron distribution and thus the effective polarity. Inductive effects and resonance can play a role here.
- Subtle Differences Magnified: Even small differences in ΔEN can lead to noticeable differences in bond properties, such as dipole moment.
Beyond Simple Electronegativity Differences: Factors Influencing Polarity
While electronegativity differences provide a valuable first-order approximation of bond polarity, other factors can also influence the actual electron distribution within a bond:
- Hybridization: The type of hybridization of the atoms involved can impact the electron density distribution. For example, the polarity of a C-H bond can vary slightly depending on whether the carbon atom is sp, sp², or sp³ hybridized.
- Resonance: In molecules with delocalized electrons (resonance structures), the electron distribution is affected by the resonance stabilization, potentially altering the overall bond polarity.
- Inductive Effects: The presence of electron-withdrawing or electron-donating groups in a molecule can influence the electron density around a particular bond, modifying its polarity.
- Steric Effects: Steric hindrance, the spatial arrangement of atoms, can also subtly affect the electron distribution and, consequently, the bond polarity.
Conclusion: A Spectrum of Polarity
The quest to identify the two most similar bonds in polarity underscores the complexity of chemical bonding. While simple comparisons based on electronegativity differences provide a useful starting point, a complete understanding requires considering the multifaceted factors influencing electron distribution. There isn't a single, universally accepted answer, as the "most similar" depends on the criteria used and the level of precision desired. The C-C and Si-Si bonds, owing to their extremely low electronegativity differences, represent a strong case for being among the most similar in terms of near nonpolarity. However, the subtle interplay of other factors highlights the continuous nature of bond polarity and the limitations of relying solely on electronegativity. Further exploration of these factors through advanced computational methods provides a more complete picture of molecular properties.
Latest Posts
Latest Posts
-
Where Is Most Of The Mass Of An Atom Concentrated
Apr 01, 2025
-
What Bone Articulates With The Acetabulum
Apr 01, 2025
-
What Is The Value Of Log Subscript 27 Baseline 9
Apr 01, 2025
-
How Many Chromosomes In Liver Cells
Apr 01, 2025
-
All Of The Following Refer To Mitosis Except
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Two Bonds Are Most Similar In Polarity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
