Explain Why Air Is A Mixture
News Leon
Mar 21, 2025 · 6 min read
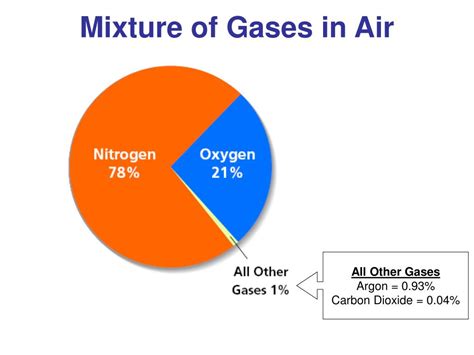
Table of Contents
Why Air is a Mixture: A Deep Dive into the Composition and Properties of Earth's Atmosphere
Air. We breathe it, we rely on it, yet many of us take its intricate composition for granted. But what exactly is air? The simple answer is: a mixture. This seemingly straightforward statement opens a door to a fascinating exploration of chemistry, physics, and the very essence of our planet's life-sustaining envelope. This article will delve deep into the reasons why air is classified as a mixture rather than a compound, examining its components, properties, and the implications of this classification.
The Defining Characteristics of Mixtures
Before delving into the specifics of air, let's establish a clear understanding of what constitutes a mixture. A mixture is a substance composed of two or more components that are not chemically bonded. This means the components retain their individual chemical properties and can be physically separated using various methods. Crucially, the relative proportions of the components in a mixture can vary. This contrasts sharply with compounds, where elements are chemically combined in fixed ratios, resulting in a substance with entirely new properties. Water (H₂O), for instance, is a compound; its properties are entirely different from those of its constituent hydrogen and oxygen.
Key features distinguishing mixtures from compounds:
- Variable composition: Mixtures can have varying proportions of their components. Air in a mountainous region will have a different composition than air at sea level.
- Retention of individual properties: Components in a mixture retain their original chemical and physical properties. The oxygen in air still behaves like oxygen, and the nitrogen retains its nitrogenous characteristics.
- Separation by physical means: Components can be separated using physical methods like distillation, filtration, or chromatography. Liquefying air and then fractional distillation is a common method to separate its components.
- No fixed ratios: Unlike compounds, there are no fixed ratios or formulas defining the composition of a mixture.
The Composition of Air: A Multi-Component System
Air is a complex mixture predominantly composed of gases, but also contains tiny particles of solids and liquids. The main components are:
1. Nitrogen (N₂): The Dominant Player
Nitrogen accounts for approximately 78% of Earth's atmosphere. While relatively inert, playing a crucial role in maintaining life is not directly as a gas, but through its incorporation into organic molecules crucial for protein synthesis. Its non-reactivity prevents uncontrolled oxidation reactions that would be harmful to life.
2. Oxygen (O₂): The Life-Sustaining Gas
Oxygen makes up about 21% of the atmosphere. Its vital role in respiration is well-known; it's essential for the metabolic processes of most living organisms. Oxygen's reactivity makes it a key element in numerous chemical processes, from combustion to rust formation.
3. Argon (Ar): The Inert Noble Gas
Argon, an inert noble gas, constitutes about 0.93% of the atmosphere. Its inertness makes it useful in various industrial applications, like welding and preventing oxidation.
4. Trace Gases: A Diverse Collection
The remaining fraction of air, about 0.07%, is composed of a multitude of trace gases, including:
- Carbon Dioxide (CO₂): Though present in small amounts (around 0.04%), CO₂ is a crucial greenhouse gas, playing a vital role in regulating Earth's temperature. Human activities have significantly increased its concentration, contributing to climate change.
- Neon (Ne), Helium (He), Methane (CH₄), Krypton (Kr), Hydrogen (H₂), and Xenon (Xe): These are present in extremely small amounts. While individually present in minuscule quantities, their collective presence showcases the complexity of air's composition.
- Water Vapor (H₂O): The amount of water vapor varies significantly depending on location and weather conditions. It is not a constant percentage like the other components.
- Ozone (O₃): Present in the stratosphere, ozone forms a protective layer that absorbs most of the sun's harmful ultraviolet radiation. Ground-level ozone, however, is a pollutant.
- Air Pollutants: These are human-made substances introduced into the atmosphere, including particulate matter, sulfur oxides, nitrogen oxides, and various volatile organic compounds. These substances vary greatly in their concentration depending on geographic location and industrial activity.
Evidence Supporting Air's Classification as a Mixture
Several pieces of evidence solidify the classification of air as a mixture:
- Variable Composition: As mentioned earlier, the composition of air varies based on location, altitude, and weather conditions. The concentration of water vapor, for instance, can fluctuate dramatically. This variability is a hallmark of mixtures.
- Physical Separation: The components of air can be physically separated using methods like fractional distillation. This involves cooling air until it liquefies and then gradually warming it, allowing the different components to boil off at their respective boiling points, thus separating them.
- Retention of Individual Properties: Each component in air maintains its chemical and physical properties. Oxygen supports combustion, nitrogen is inert, and argon remains unreactive, just as they would in their pure forms.
- No Fixed Ratio: The proportions of gases in air are not fixed. The concentration of CO₂, for example, has been steadily increasing due to human activities, highlighting the lack of a fixed ratio in air’s composition.
The Implications of Air Being a Mixture
The fact that air is a mixture has significant implications for our understanding of the atmosphere and its role in supporting life on Earth:
- Environmental Monitoring: Understanding air's composition is crucial for monitoring air quality and identifying pollutants. This allows for the development of strategies to mitigate air pollution and protect human health.
- Climate Change Research: The variable composition of air, especially the changing concentrations of greenhouse gases, is central to climate change research. Accurate measurements and models of air composition are vital for understanding and predicting climate change impacts.
- Industrial Applications: The separation of air's components has numerous industrial applications. Oxygen is used in healthcare and various industries, while nitrogen is used in food preservation and other applications. Argon is used in welding and other processes.
- Life Support Systems: Understanding air's composition is critical for designing life support systems for astronauts, submarines, and other enclosed environments. These systems must carefully control the levels of various gases to ensure human survival.
Conclusion: A Dynamic and Vital Mixture
In conclusion, the evidence overwhelmingly supports the classification of air as a mixture. Its variable composition, the ability to separate its components physically, and the retention of individual properties by its constituents all point to this conclusion. Air is not merely a collection of gases; it's a dynamic and incredibly complex system, a life-sustaining mixture whose delicate balance is essential for the existence of life on Earth. Understanding the intricacies of this mixture is fundamental to addressing crucial environmental challenges and harnessing its properties for various technological advancements. From the microscopic level of molecular interactions to the global scale of atmospheric processes, the study of air continues to unveil fascinating insights into the functioning of our planet and the delicate web of life that it supports. Further research and advancements in atmospheric science promise to provide an even deeper understanding of this vital and complex mixture that we depend on for survival.
Latest Posts
Latest Posts
-
Draw 10 Water Molecules To Create A Cluster
Mar 22, 2025
-
Half Of 1 And 1 2 Teaspoons
Mar 22, 2025
-
Number Of Cells In The Interphase
Mar 22, 2025
-
Which Part Of The Digestive System Primarily Absorbs Water
Mar 22, 2025
-
Which Of These Molecular Electron Configurations Describe An Excited State
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Explain Why Air Is A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
