Draw 10 Water Molecules To Create A Cluster
News Leon
Mar 22, 2025 · 7 min read
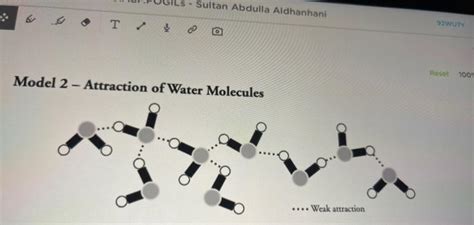
Table of Contents
Drawing 10 Water Molecules to Create a Cluster: A Deep Dive into Hydrogen Bonding and Water's Unique Properties
Water. It's the elixir of life, the solvent of countless reactions, and a substance so fundamental to our existence that we often take its remarkable properties for granted. One key aspect of water's unique behavior stems from its molecular structure and the way individual water molecules interact with one another to form clusters. This article explores the intricacies of drawing ten water molecules to create a cluster, delving into the underlying principles of hydrogen bonding and the implications for various scientific fields.
Understanding the Water Molecule: A Foundation for Clustering
Before we embark on the artistic and scientific endeavor of drawing a water cluster, let's revisit the fundamental building block: the water molecule itself. Represented chemically as H₂O, a single water molecule consists of one oxygen atom covalently bonded to two hydrogen atoms. This arrangement isn't linear; instead, it forms a bent structure with a bond angle of approximately 104.5 degrees. This bent geometry plays a crucial role in the formation of hydrogen bonds, the driving force behind water clustering.
The Polar Nature of Water: A Key to Interaction
Oxygen is significantly more electronegative than hydrogen. This means it attracts the shared electrons in the covalent bonds more strongly, creating a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the hydrogen atoms. This polarity is what makes water a polar molecule, capable of forming strong intermolecular interactions with other water molecules and other polar substances.
Hydrogen Bonding: The Glue that Holds Water Clusters Together
Hydrogen bonding is a special type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. In the case of water, the partially positive hydrogen atom of one water molecule is attracted to the partially negative oxygen atom of a neighboring water molecule.
This attraction isn't as strong as a covalent bond, but it's significantly stronger than other intermolecular forces, like van der Waals forces. The strength of these hydrogen bonds is responsible for many of water's unique properties, including its high boiling point, high surface tension, and ability to act as an excellent solvent.
Visualizing Hydrogen Bonds: Drawing the Connections
When drawing water molecules, it's crucial to represent these hydrogen bonds explicitly. Typically, a dashed or dotted line is used to indicate the hydrogen bond between the partially positive hydrogen atom of one molecule and the partially negative oxygen atom of another. This visual representation helps convey the interconnectedness of the molecules within the cluster.
Drawing Ten Water Molecules: A Step-by-Step Approach
Now, let's tackle the challenge of drawing ten water molecules to create a cluster. There's no single "correct" way to do this, as the arrangement of molecules in a water cluster is dynamic and constantly changing. However, the following steps offer a structured approach:
-
Start with a Central Molecule: Begin by drawing a single water molecule, representing the oxygen atom with a larger circle and the hydrogen atoms with smaller circles. Ensure the bent structure is clearly visible.
-
Add Neighboring Molecules: Strategically add additional water molecules, focusing on maximizing the number of hydrogen bonds. Each water molecule should ideally have at least two hydrogen bonds to other molecules in the cluster.
-
Represent Hydrogen Bonds: Use dashed or dotted lines to represent the hydrogen bonds between molecules. Clearly indicate the interaction between the partially positive hydrogen and the partially negative oxygen.
-
Three-Dimensional Representation (Optional): For a more accurate representation, consider a three-dimensional drawing. This can be achieved by using perspective or employing shading to suggest depth.
-
Iteration and Refinement: Experiment with different arrangements of the molecules, aiming for a structure that maximizes hydrogen bonding. The cluster won't be perfectly symmetrical; instead, it will likely have a somewhat irregular and dynamic shape.
-
Labeling (Optional): For clarity, you can label the oxygen and hydrogen atoms in a few key molecules to reinforce the understanding of the molecular structure.
-
Consider the Dynamics: Remember that the cluster isn't static. The hydrogen bonds are constantly breaking and reforming, leading to a dynamic, fluid structure.
The Importance of Water Clusters: Implications Across Disciplines
The study of water clusters isn't merely an academic exercise; it holds significant implications across various scientific and technological fields:
Atmospheric Science: Cloud Formation and Precipitation
Water clusters play a crucial role in atmospheric processes, particularly cloud formation and precipitation. The initial stages of cloud formation involve the aggregation of water molecules into larger clusters around atmospheric aerosols (tiny particles in the air). These clusters then grow in size through condensation, eventually forming cloud droplets and, ultimately, precipitation. Understanding the dynamics of water clusters is essential for improving weather prediction models and climate change research.
Biology: The Role of Water in Biological Systems
Water is fundamental to life, acting as a solvent for biological molecules, a reactant in numerous biochemical reactions, and a component of various cellular structures. Water clusters play a key role in the folding and function of proteins, influencing their interactions with other molecules. The structure and dynamics of water clusters within biological systems are areas of ongoing research.
Chemistry: Solvation and Reactivity
Water's ability to act as a universal solvent arises from its ability to form hydrogen bonds with a wide range of molecules. The solvation of ions and polar molecules in water often involves the formation of water clusters around the solute, affecting its reactivity and transport properties. Understanding water cluster dynamics is crucial for understanding chemical reactions in aqueous solutions.
Materials Science: Novel Materials and Applications
The unique properties of water clusters have led to exploring their use in various materials science applications. For example, researchers are investigating the potential of water clusters to enhance the performance of fuel cells, batteries, and other energy-related technologies. Furthermore, understanding water-material interactions is crucial for designing new materials with specific properties.
Nanotechnology: Controlled Assembly and Manipulation of Water Clusters
Recent advances in nanotechnology have allowed scientists to manipulate water clusters at the nanoscale, opening new possibilities for designing nanoscale devices and materials. The ability to control the size and shape of water clusters could have significant implications for various technological applications.
Further Exploration: Beyond Ten Molecules
While drawing ten water molecules provides a good starting point for understanding water clustering, the complexity of these structures increases significantly as the number of molecules grows. Larger clusters exhibit a wider range of structures and dynamics, leading to a richer understanding of water's properties.
Computational Modeling: Simulating Water Cluster Dynamics
Computational techniques, such as molecular dynamics simulations, are essential tools for studying the behavior of large water clusters. These simulations provide valuable insights into the structure, dynamics, and properties of these clusters that are impossible to obtain through experimental methods alone.
Experimental Techniques: Probing Water Cluster Structure and Dynamics
Experimental techniques, like X-ray diffraction, neutron scattering, and spectroscopy, provide crucial information about the structure and dynamics of water clusters. These methods offer complementary insights to computational studies and contribute to a more complete understanding of these fascinating systems.
Conclusion: The Enduring Mystery and Significance of Water Clusters
Drawing ten water molecules to create a cluster is a starting point to appreciate the intricate dance of hydrogen bonds and the remarkable properties of water. Understanding water cluster dynamics is not just an academic pursuit; it holds profound implications for various scientific and technological fields. As research progresses, we can expect further breakthroughs in our understanding of these fascinating molecular assemblies and their role in shaping our world. The enduring mystery of water, its simple yet profound nature, continues to inspire and challenge scientists worldwide.
Latest Posts
Latest Posts
-
A Part Of A Line That Has Two Endpoints
Mar 22, 2025
-
Which Of The Following Is A Prokaryotic Cell
Mar 22, 2025
-
Sulfuric Acid With Sodium Hydroxide Balanced Equation
Mar 22, 2025
-
A Rectangle Inscribed In A Semicircle Of Radius R
Mar 22, 2025
-
Which Subatomic Particle Is The Heaviest
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Draw 10 Water Molecules To Create A Cluster . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
