Sulfuric Acid With Sodium Hydroxide Balanced Equation
News Leon
Mar 22, 2025 · 6 min read
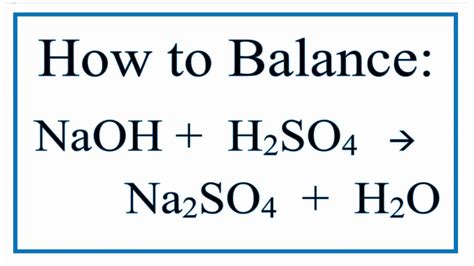
Table of Contents
Sulfuric Acid and Sodium Hydroxide: A Deep Dive into the Neutralization Reaction
The reaction between sulfuric acid (H₂SO₄) and sodium hydroxide (NaOH) is a classic example of a neutralization reaction, a fundamental concept in chemistry. Understanding this reaction requires exploring the properties of each reactant, the balanced chemical equation, the stoichiometry involved, and the applications of this reaction in various fields. This comprehensive article will delve into all these aspects, providing a thorough understanding of the sulfuric acid and sodium hydroxide reaction.
Understanding the Reactants: Sulfuric Acid and Sodium Hydroxide
Before examining the reaction itself, let's individually explore the properties of sulfuric acid and sodium hydroxide.
Sulfuric Acid (H₂SO₄)
Sulfuric acid, also known as vitriol, is a highly corrosive strong mineral acid. Its properties include:
- Strong Acidity: It readily donates protons (H⁺ ions) in aqueous solutions, leading to a high concentration of hydronium ions (H₃O⁺). This contributes to its highly acidic nature and corrosive properties.
- Diprotic Acid: Sulfuric acid is a diprotic acid, meaning it has two acidic protons that can be donated in a stepwise manner. This leads to two distinct neutralization stages with a base like sodium hydroxide.
- Dehydrating Agent: Concentrated sulfuric acid is a potent dehydrating agent, capable of removing water molecules from other substances. This property finds applications in various industrial processes.
- Oxidizing Agent: Concentrated sulfuric acid can act as an oxidizing agent, particularly at elevated temperatures, reacting with certain metals and reducing agents.
Sodium Hydroxide (NaOH)
Sodium hydroxide, commonly known as caustic soda or lye, is a strong alkali. Key properties include:
- Strong Basicity: It readily accepts protons (H⁺ ions) in aqueous solutions, forming hydroxide ions (OH⁻). This results in a high pH value and strongly alkaline nature.
- Highly Corrosive: Similar to sulfuric acid, sodium hydroxide is highly corrosive and can cause severe chemical burns upon contact with skin or eyes.
- Hygroscopic Nature: Sodium hydroxide absorbs moisture from the air, making it deliquescent. This property necessitates proper storage to prevent contamination.
- Use in Various Industries: Sodium hydroxide is widely used in various industries, including the production of soap, paper, textiles, and detergents.
The Balanced Chemical Equation: A Step-by-Step Explanation
The reaction between sulfuric acid and sodium hydroxide is a neutralization reaction that produces sodium sulfate and water. The balanced chemical equation represents this process accurately, ensuring the law of conservation of mass is obeyed. The complete reaction occurs in two steps:
Step 1:
H₂SO₄(aq) + NaOH(aq) → NaHSO₄(aq) + H₂O(l)
In this first step, one mole of sulfuric acid reacts with one mole of sodium hydroxide to form one mole of sodium bisulfate (NaHSO₄), also known as sodium hydrogen sulfate, and one mole of water. This is a partial neutralization, as one proton from the sulfuric acid has been neutralized.
Step 2:
NaHSO₄(aq) + NaOH(aq) → Na₂SO₄(aq) + H₂O(l)
The second step involves the neutralization of the remaining acidic proton in the sodium bisulfate. One mole of sodium bisulfate reacts with another mole of sodium hydroxide to yield one mole of sodium sulfate (Na₂SO₄) and another mole of water.
Overall Balanced Equation:
Combining the two steps, the overall balanced chemical equation for the complete neutralization of sulfuric acid with sodium hydroxide is:
H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l)
This equation indicates that one mole of sulfuric acid reacts with two moles of sodium hydroxide to produce one mole of sodium sulfate and two moles of water. The (aq) denotes aqueous solutions, while (l) represents liquid water.
Stoichiometry and Calculations: Understanding Mole Ratios
The balanced chemical equation provides crucial information about the stoichiometry of the reaction. It reveals the mole ratios of reactants and products. For instance, the mole ratio of sulfuric acid to sodium hydroxide is 1:2. This means that for every one mole of sulfuric acid, two moles of sodium hydroxide are required for complete neutralization. This ratio is essential for performing stoichiometric calculations to determine the amount of reactants needed or products formed in a given reaction.
For example, if you have 0.5 moles of sulfuric acid, you would need 1 mole of sodium hydroxide (0.5 moles * 2 = 1 mole) for complete neutralization. Conversely, if you have 2 moles of sodium hydroxide, you would only need 1 mole of sulfuric acid for complete reaction.
Applications of the Sulfuric Acid and Sodium Hydroxide Reaction
This seemingly simple neutralization reaction has numerous applications across various industries and fields. Some notable examples include:
- Industrial Processes: The reaction is used in various industrial processes to control pH levels, neutralize acidic or alkaline waste streams, and in the production of sodium sulfate, a vital chemical used in detergents and other products.
- Chemical Synthesis: The reaction is a crucial step in various chemical syntheses where precise pH control is necessary. It's used as a method to prepare pure sodium sulfate.
- Wastewater Treatment: In wastewater treatment plants, sulfuric acid and sodium hydroxide are used to adjust the pH of wastewater to meet environmental regulations before discharge. Neutralizing acidic or alkaline wastewater prevents environmental damage.
- Titration: This reaction is frequently used in acid-base titrations to determine the concentration of either sulfuric acid or sodium hydroxide solutions. The precise stoichiometry allows accurate determination of the unknown concentration.
Safety Precautions: Handling Corrosive Chemicals
Both sulfuric acid and sodium hydroxide are highly corrosive chemicals that require careful handling. It is crucial to always follow safety guidelines when working with these substances:
- Protective Gear: Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, lab coats, and closed-toe shoes.
- Ventilation: Ensure adequate ventilation to prevent inhalation of fumes. The reaction can generate heat, so take appropriate precautions.
- Slow Addition: When mixing the two solutions, always add the acid or base slowly to the other, stirring constantly to prevent splashing and heat buildup. Adding acid to water is generally recommended for dilution.
- Emergency Procedures: Have a readily available eyewash station and safety shower nearby in case of accidents. Know the emergency procedures for chemical spills.
Beyond the Basics: Exploring Related Reactions and Concepts
Understanding the sulfuric acid and sodium hydroxide reaction opens doors to exploring more advanced concepts:
- Acid-Base Titrations: The reaction serves as a foundation for acid-base titrations, a quantitative analytical technique widely used in chemistry.
- pH and Buffers: This reaction's understanding helps in comprehending pH changes and the role of buffers in maintaining a stable pH in solutions.
- Thermochemistry: The reaction itself involves heat transfer, leading to an exploration of thermochemical principles like enthalpy changes and heat capacity.
- Equilibrium Constants: The reaction equilibrium and its associated equilibrium constant (K) can be explored, understanding the factors influencing reaction direction.
Conclusion: A Fundamental Reaction with Wide-Reaching Implications
The neutralization reaction between sulfuric acid and sodium hydroxide is a seemingly simple yet fundamental chemical process with far-reaching implications in diverse fields. From industrial applications to chemical analysis, understanding this reaction's stoichiometry, safety precautions, and broader implications is crucial for anyone working in science, engineering, or related fields. This reaction serves as an excellent example of a fundamental chemical principle with significant practical applications, showcasing the power of chemistry to address diverse challenges. Continued exploration of this reaction and its associated concepts will enhance your understanding of chemical principles and their practical relevance.
Latest Posts
Latest Posts
-
How Many Corners Does A Cuboid Have
Mar 23, 2025
-
How To Write Letter To The Bank
Mar 23, 2025
-
How Long Does A Cow Sleep
Mar 23, 2025
-
A Skier Is Pulled By A Tow Rope
Mar 23, 2025
-
Is A Candle Burning A Physical Or Chemical Change
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Sulfuric Acid With Sodium Hydroxide Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
