Which Of These Molecular Electron Configurations Describe An Excited State
News Leon
Mar 22, 2025 · 5 min read
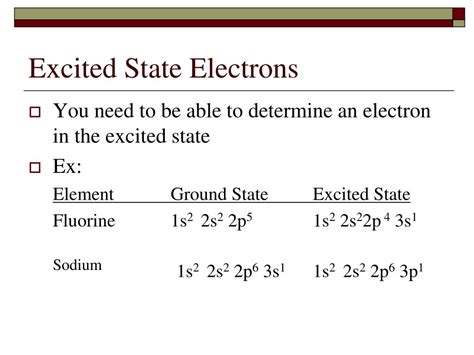
Table of Contents
Which of These Molecular Electron Configurations Describe an Excited State?
Understanding molecular electron configurations is crucial for comprehending the behavior of molecules, their reactivity, and their spectroscopic properties. A key aspect of this understanding is the ability to differentiate between ground states and excited states. This article delves into the intricacies of molecular electron configurations, focusing on how to identify excited states. We will explore various examples and provide a comprehensive framework for determining which configurations represent an excited state.
Understanding Ground States and Excited States
Before diving into specific examples, let's establish a clear definition of ground and excited states.
-
Ground State: The ground state is the lowest energy state of a molecule. All electrons occupy the lowest available molecular orbitals (MOs) consistent with the Pauli Exclusion Principle (which states that no two electrons can have the same four quantum numbers) and Hund's Rule (which states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital). This configuration is the most stable and represents the molecule's normal state.
-
Excited State: An excited state occurs when one or more electrons are promoted to higher energy molecular orbitals. This transition requires energy input, typically in the form of light absorption. Excited states are inherently less stable than the ground state and will eventually decay back to the ground state, often releasing energy in the form of light emission (fluorescence or phosphorescence).
Identifying Excited States: A Step-by-Step Approach
Identifying excited states involves systematically analyzing the electron configuration relative to the ground state configuration. Here's a step-by-step approach:
-
Determine the total number of valence electrons: Count the valence electrons contributed by each atom in the molecule. This is crucial for correctly filling the molecular orbitals.
-
Construct the Molecular Orbital Diagram: Draw a molecular orbital (MO) diagram for the molecule. This diagram shows the relative energy levels of the MOs and how the valence electrons are distributed. The order of energy levels can vary depending on the molecule (e.g., diatomic molecules vs. polyatomic molecules). Common MOs include bonding orbitals (σ, π) and antibonding orbitals (σ*, π*).
-
Fill the Molecular Orbitals: Fill the MOs according to the Aufbau principle (electrons fill the lowest energy levels first), the Pauli exclusion principle, and Hund's rule. This will give you the ground state electron configuration.
-
Compare to the Given Configuration: Compare the given electron configuration to the ground state configuration you've just determined. If any electrons occupy higher-energy orbitals than in the ground state, the configuration represents an excited state.
-
Analyze Electron Promotion: Identify which electrons have been promoted to higher energy levels. This will help you understand the nature of the excitation.
Examples: Identifying Excited States
Let's illustrate this process with a few examples. We'll consider hypothetical molecular electron configurations for diatomic molecules, keeping it simplified for clarity. Remember, actual MO diagrams and energy level orderings can become quite complex for larger molecules.
Example 1:
Let's consider a hypothetical diatomic molecule with 10 valence electrons.
- Ground State Configuration: (σ₂s)²(σ₂s*)²(σ₂p)²(π₂p)⁴
- Hypothetical Configuration A: (σ₂s)²(σ₂s*)²(σ₂p)²(π₂p)³(π₂p*)¹
- Hypothetical Configuration B: (σ₂s)²(σ₂s*)²(σ₂p)²(π₂p)⁴(σ₂p*)²
Analysis:
-
Configuration A: One electron has been promoted from a π₂p bonding orbital to a π₂p* antibonding orbital. This clearly represents an excited state.
-
Configuration B: Two electrons have been promoted from the π₂p bonding orbitals to the σ₂p* antibonding orbital. This also represents an excited state, with a significantly higher energy than Configuration A.
Example 2: A More Complex Scenario
Consider a hypothetical triatomic molecule with 14 valence electrons. Let's assume a simplified MO diagram with the following energy levels (from lowest to highest): σ₁, σ₂, π₁, π₂*, σ₃*, π₃*.
-
Ground State Configuration (Hypothetical): (σ₁)²(σ₂)²(π₁)²(π₁)²
-
Hypothetical Configuration C: (σ₁)²(σ₂)²(π₁)²(π₂*)¹(π₁)²
Analysis:
- Configuration C: One electron is promoted from a π₁ bonding orbital to a π₂* antibonding orbital. This represents an excited state. The complexity arises from the presence of multiple π orbitals and the need to accurately assign electrons to the correct levels based on the assumed energy order.
Factors Influencing Excited State Characteristics
Several factors influence the characteristics of excited states:
-
Energy of Excitation: The energy difference between the ground state and the excited state determines the energy of the photon required to cause the transition. Higher energy transitions result in higher energy photons (e.g., UV light).
-
Lifetime of Excited State: Excited states are inherently unstable and will decay back to the ground state. The lifetime of an excited state varies greatly, depending on the molecule and the specific excited state.
-
Decay Mechanisms: Excited states can decay through several mechanisms, including fluorescence (emission of a photon at a lower energy), phosphorescence (similar to fluorescence but with a longer lifetime), or non-radiative decay (conversion of excess energy into vibrational energy).
-
Molecular Geometry: The geometry of a molecule can influence both its ground state and excited state configurations. Excited state geometries may differ significantly from the ground state geometry.
Applications of Excited State Knowledge
Understanding excited states is crucial in various fields, including:
-
Spectroscopy: Spectroscopic techniques, such as UV-Vis and fluorescence spectroscopy, rely on the excitation and subsequent decay of molecules to study their structure and properties.
-
Photochemistry: Photochemical reactions involve the use of light to initiate chemical reactions. Understanding excited states is fundamental to designing and controlling such reactions.
-
Materials Science: Excited states play a vital role in the properties of many materials, including luminescent materials, semiconductors, and photovoltaic devices.
Conclusion
Determining whether a molecular electron configuration describes an excited state requires a systematic approach involving constructing a molecular orbital diagram, filling the orbitals according to the rules of electron configuration, and comparing the result to the ground state configuration. By carefully analyzing electron promotion to higher energy levels, we can accurately identify excited states and understand their importance in molecular behavior and applications across diverse scientific disciplines. Remember that the complexity of determining excited states increases with the size and complexity of the molecule, often requiring computational methods for accurate predictions. However, understanding the fundamental principles remains essential for interpreting spectroscopic data and understanding molecular reactivity.
Latest Posts
Latest Posts
-
Complete The Sentence With The Correct Form Of The Word
Mar 22, 2025
-
What Causes Movement Along The Demand Curve
Mar 22, 2025
-
A Cat Dozes On A Stationary Merry Go Round
Mar 22, 2025
-
What Is The Name For N2o5
Mar 22, 2025
-
An Isolated Conductor Has A Net Charge Of
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Which Of These Molecular Electron Configurations Describe An Excited State . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
