Electrons Involved In Bonding Between Atoms Are
News Leon
Mar 16, 2025 · 6 min read
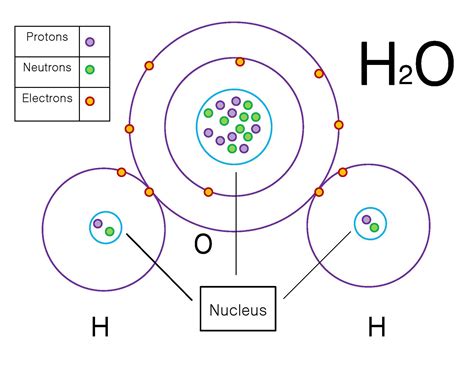
Table of Contents
Electrons Involved in Bonding Between Atoms: A Deep Dive into Chemical Bonding
Chemical bonding, the force that holds atoms together to form molecules and compounds, is fundamentally driven by the behavior of electrons. Understanding how electrons participate in bonding is key to comprehending the properties and behavior of matter. This article delves into the intricate world of electron involvement in various types of chemical bonds, exploring ionic, covalent, and metallic bonding in detail.
The Role of Valence Electrons
Before diving into specific bond types, it's crucial to understand the concept of valence electrons. These are the electrons located in the outermost shell (or energy level) of an atom. They are the primary players in chemical bonding because they experience the least electrostatic attraction from their own nucleus and are therefore more readily available for interaction with other atoms. The number of valence electrons an atom possesses dictates its bonding capacity and chemical reactivity. Elements in the same group (vertical column) of the periodic table share the same number of valence electrons and exhibit similar chemical behavior.
For instance, elements in Group 1 (alkali metals) like lithium (Li) and sodium (Na) have one valence electron, making them highly reactive. In contrast, elements in Group 18 (noble gases) like helium (He) and neon (Ne) possess a full valence shell (2 electrons for He, 8 for others), rendering them exceptionally stable and unreactive. This stability is the driving force behind the formation of many chemical bonds, as atoms strive to achieve a stable electron configuration, often resembling that of a noble gas.
Ionic Bonding: The Transfer of Electrons
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. This type of bonding occurs when one atom transfers one or more valence electrons to another atom. The atom that loses electrons becomes a positively charged ion (cation), while the atom that gains electrons becomes a negatively charged ion (anion).
The driving force behind ionic bonding is the achievement of a stable octet (eight valence electrons) by both ions. This is particularly common with metals (which readily lose electrons) and nonmetals (which readily gain electrons). For example, in the formation of sodium chloride (NaCl, common table salt), sodium (Na) loses one valence electron to chlorine (Cl), forming Na⁺ and Cl⁻ ions respectively. The strong electrostatic attraction between these oppositely charged ions creates the ionic bond.
Factors influencing ionic bond strength:
- Charge of the ions: Higher charges lead to stronger attraction. For example, the bond in MgO (Mg²⁺ and O²⁻) is stronger than in NaCl (Na⁺ and Cl⁻).
- Size of the ions: Smaller ions result in stronger attraction because the positive and negative charges are closer together.
- Lattice energy: The energy released when gaseous ions form a solid crystal lattice is a measure of ionic bond strength. Higher lattice energy indicates a stronger bond.
Properties of ionic compounds:
Ionic compounds typically exhibit high melting and boiling points due to the strong electrostatic forces between ions. They are often brittle and crystalline in nature. When dissolved in water, they conduct electricity because the ions become mobile and can carry an electric current.
Covalent Bonding: The Sharing of Electrons
Covalent bonds involve the sharing of one or more pairs of valence electrons between two atoms. This type of bonding is prevalent between nonmetal atoms, which generally have high electronegativity (a measure of an atom's ability to attract electrons in a chemical bond).
Instead of transferring electrons, atoms in a covalent bond share electrons to achieve a stable electron configuration, usually an octet. The shared electrons are attracted to the nuclei of both atoms, holding them together. The shared electron pair forms a chemical bond, often represented by a single line (-) in Lewis structures. Multiple bonds (double bonds, triple bonds) can form when two or more pairs of electrons are shared.
Types of covalent bonds:
- Nonpolar covalent bonds: Occur when electrons are shared equally between two atoms of similar electronegativity. Examples include the bonds in diatomic molecules like H₂, O₂, and N₂.
- Polar covalent bonds: Occur when electrons are shared unequally between two atoms with different electronegativities. The atom with higher electronegativity attracts the shared electrons more strongly, creating a partial negative charge (δ⁻) on that atom and a partial positive charge (δ⁺) on the other atom. Water (H₂O) is a classic example, with oxygen being more electronegative than hydrogen.
Properties of covalent compounds:
Covalent compounds often have lower melting and boiling points compared to ionic compounds because the intermolecular forces (forces between molecules) are generally weaker than the electrostatic forces in ionic compounds. They can exist as gases, liquids, or solids at room temperature, depending on the strength of the intermolecular forces. They generally do not conduct electricity in their pure state but may conduct electricity when dissolved in certain solvents, if they ionize.
Metallic Bonding: A Sea of Electrons
Metallic bonding is a unique type of bonding found in metals. In contrast to ionic and covalent bonds, metallic bonding does not involve the transfer or sharing of electrons in discrete pairs. Instead, valence electrons are delocalized and form a "sea" of electrons that surrounds positively charged metal ions.
These delocalized electrons are not associated with any particular atom but are free to move throughout the metal lattice. This mobility of electrons is responsible for many characteristic properties of metals, such as their high electrical and thermal conductivity, malleability (ability to be hammered into sheets), and ductility (ability to be drawn into wires).
Factors affecting metallic bond strength:
- Number of valence electrons: More valence electrons contribute to a stronger metallic bond, as more electrons are available to participate in the electron sea.
- Size of the metal ions: Smaller ions lead to stronger metallic bonds because the positively charged ions are closer to the electron sea, resulting in stronger electrostatic attraction.
Properties of metallic compounds:
Metals are typically lustrous, malleable, ductile, and good conductors of heat and electricity. Their melting points and boiling points vary greatly depending on the metal and the strength of the metallic bond. The unique properties of metallic bonding enable the use of metals in a wide range of applications, from construction materials to electronic components.
Beyond the Basics: More Complex Bonding Scenarios
The three main types of bonding (ionic, covalent, and metallic) represent idealized models. In reality, many compounds exhibit characteristics of more than one type of bonding. For example, some compounds exhibit polar covalent character, where the electron distribution is uneven, resulting in partial charges on the atoms.
Furthermore, the concept of resonance is essential for understanding the bonding in certain molecules. Resonance describes a situation where a molecule can be represented by multiple Lewis structures that differ only in the placement of electrons. The actual molecule is a hybrid of these resonance structures, with electron density distributed over multiple atoms. Benzene (C₆H₆) is a classic example of a molecule with resonance structures.
Hydrogen bonding, a special type of intermolecular force, is significantly stronger than other intermolecular forces and plays a crucial role in the properties of many molecules, including water. It occurs when a hydrogen atom covalently bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule.
The fascinating interplay of electrons in chemical bonds underpins the vast diversity of matter in the universe. Understanding these fundamental principles allows us to predict and explain the properties of various substances and is crucial in fields ranging from materials science to biochemistry. Continuous research continues to refine our understanding of chemical bonding and its complexities. The study of electron behavior in chemical bonds remains an active area of research, pushing the boundaries of our knowledge and enabling innovative technological advancements.
Latest Posts
Latest Posts
-
Which Of The Following Is Polynomial
Mar 17, 2025
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Electrons Involved In Bonding Between Atoms Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
